Hydroxyl compounds
advertisement

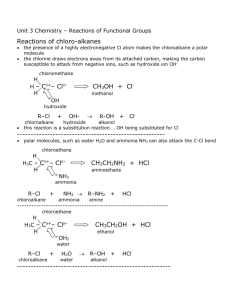
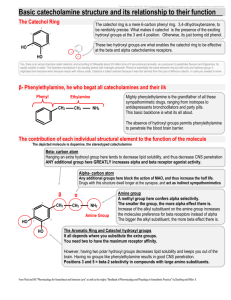
Hydroxyl compounds A. Classification: 1. alkanol: primary alcohol (1 alkanol) butan-1-ol OH OH secondary alcohol (2 alkanol) tertiary alcohol (3 alkanol) butan-2-ol 2-methylbutan-2-ol OH OH 2. phenol Classify by number of hydoxyl groups per molecule: 1. monohydric alkanol H H H ethanol H H O H O 2. polyhydric alkanol H H ethane-1,2-diol O H HH H B. IUPAC name: Br OH OH pent-4-en-2-ol 3-bromocyclohexanol OH but-3-yn-1-ol OH OH OH HO OH hex-2-ene-1,6-diol 2-methylphenol o-methylphenol 1,4-dihydroxybenzene p-dihydroxybenzene C. Preparation: Industrial process: 1. Fermentation of carbohydrates: yeast C6 H12O6 (aq) 2CO2 ( g ) 2CH 3CH 2OH (aq) 2. Hydration of alkene: Hydroxyl groups / page 1 H H H H + H O HgSO 4 H O H H SO 2 4 O H H H H H 3. Formation of methanol: _ pressure, 400 C , Cr2 O3 ZnO CO( g ) 2 H 2 ( g ) high CH3OH o Laboratory method: 1. Nucleophilic substitution: + OH + Br - HO Br 2. Reduction of carbonyl compounds a. By lithium tetrahydrioaluminate LiAlH4: Li AlH4 in ether is a strong reducing agent which can reduce acid, ester, carbonyl group (containing O atom) but not alkene and alkyne. 1. LiAlH /ether 4 + 2. H OH O b. By hydrogenation: Hydrogenation can also be used to convert alkene and alkyne to alkane. Pt + H O 2 OH 3. Oxidation of alkenes: Oxidize alkene by strong oxidizing agent, acidified potassium permanganate. OH + H O H + (O) KM nO /H 4 + OH Phenol: 1. Sulphonation O S SO OH ONa NaOH O 3 H OH + o 400 C H SO 2 4 2. Diazo salt O N HNO 3 O H SO 2 4 H O H Zn HCl NH2 + N N NaNO3 HCl OH 3. Physical properties of alkanol: Hydroxyl groups / page 2 a. Intermolecular H-bond b. High boiling point, melting point and solubility in water 4. Chemical properties of alkanol: Principle of reaction: ROH Breakage of OH bond to form RO ion favor 1alkanol or R with electron withdrawing group Breakage of CO bond to form C+ ion Favour stable carbonium ion a. Intramolecular dehydration (Elimination, alkene formation) By dehrating agent: Condition: high temperature 180C, Conc. sulphuric acid, mole ratio of alkanol:acid = 1:1 H 2 SO OH 4 + H 180 o C O H By catalyst: Condition: Aluminium oxide as catalyst, 350C Al O 3 2 OH + o H O H 350 C b. Intermolecular dehydration Condition: high temperature 140C, Conc. sulphuric acid, mole ratio of alkanol:acid = 2:1 H 2 SO 4 + OH H + 140o C HO O O H c. Halogenation Lucas Test: R H O H Cl + H Cl ZnCl R Cl + H O H 2 Reagent: zinc chloride + Conc. HCl Procedure: Mix the Lucas reagent with alkanol; observe the rate of the appearance of the turbidity. (Chloroalkane oil droplets are insoluble in aqueous phase.) Result: Turbidity forms immediately. Turbidity forms slowly (5 minutes). 3 alkanol 2 alkanol Turbidity does not form. Other reagents for halogenation: PX3, PX5, SOCl2 1 alkanol PX 3 3ROH 3RX H 3 PO3 PX 5 ROH RX POX 3 HX white fumes SOCl2 ROH RCl SO2 HCl d. React with sodium Hydroxyl groups / page 3 2CH 3OH 2 Na 2 NaOCH 3 H 2 sodium methoxide, a strong organic alkali Application of sodium alkoxide: Formation of ether: + Na O + NaBr NaBr + O Br Formation of alkene: Br + Na O + OH e. Esterification (Condensation) O O conc. H SO 4 2 + OH + O H O H HO ethyl ethanoate f. Oxidation Oxidizing agents: KMnO4/H+/neutral/OH Mild oxidizing agent: CuO + KMnO4 /H + O + (O) H O H OH aldehyde Strong oxidizing agent: KMnO4/H+ + KMnO /H 4 O O (O) + OH acid Different alkanols are refluxed with acidified potassium permanganate: Primary alkanol: O + KMnO / H 4 OH + OH + 2 (O) H O H Secondary alkanol: + OH (O) + KMnO / H 4 + HOH O ketone Tertiary alkanol: Hydroxyl groups / page 4 KM nO / H 4 + no change (O) + OH g. Haloform reaction (Iodoform test) O I + I + NaOH 2 2 CHI NaOH OH 3 - + O Na + O iodoform, yellow solid 5. Physical properties of phenol: Colouless solid (slightly pink) at room temperature, high vapour pressure at room temperature, slightly soluble in water, highly toxic, high melting point and boiling point due to intermolecular hydrogen bonding 6. Chemical properties of phenol: a. Acidic O OH H + + phenoxide ion Reasons: O in phenol becomes slightly electron deficient + O OH + O H + O H H - - Phenoxide ion is stabilized by resonance effect O - - O O - O - React with NaOH: - + O Na OH + NaOH + NaHCO + H O H OH no change 3 b. Act as nucleophile in nucleophilic substitution Hydroxyl groups / page 5 O Cl O Na + + NaCl + NaCl O O Na O + O Cl O O O Na + O + Na O O O O c. Electrophilic substitution Phenol is an activating group, o,p-directing, explain by resonance effect Nitration: OH OH OH dil. HNO 3 + H SO 2 4 O O N N O O major O O N OH conc. HNO OH 3 O H SO 2 4 O N N O O 2,4,6-trinitrophenol (a kind of explosive) Halogenation: Br OH OH Br Br Br Br a product in the experiment involving rate order Sulphonation: OH co nc. H2 SO4 SO OH O 3 S OH O benzenesulphonic acid d. Complex formation React with iron (III) ion to form a blue/violet coloration. Hydroxyl groups / page 6 OH OH Fe 3+ + n Fe ( ) 3+ n Hydroxyl groups / page 7