Review - USD305.com
advertisement

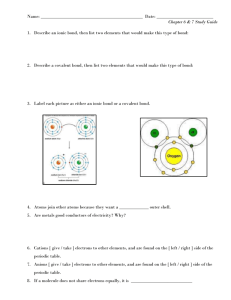
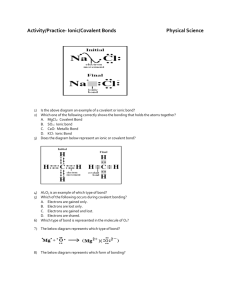
Name: ___________________ Class: ___________ Unit 5 Test Review 1. How many valence electrons are in each of the following atoms AND what are their charges when they form ions: a) P b) Ca c) B 2. Define: a) Valence electron – b) Ionic Bondc) Covalent Bondd) Unshared (Lone) Pair – e) VSEPR – f) Resonance Structure – g) Subscript – h) Polar – i) Nonpolar – j) Hydrogen bond – k) Dispersion force – l) Dipole-dipole interaction – 3. What is the overall charge of any ionic compound? 4. Which subatomic particles are free to drift in metallic compounds? 5. Circle all of the elements below that are diatomic. O Ar H N U Zn Cl 6. What do the following elements do to gain an octet (become stable) a) Mg b) S 7. Fill in each of the blanks below with the word metal or nonmetal. a. An ionic compound is made of a ______________ and a ________________. b. A covalent compound is made of ______________________. c. A metallic bond is made of _______________________. 9. Write I if the compound is ionic and C if the compound is covalent a. CCl4 _______ b. KCl _______ c. CuI _______ 10. What are the properties of ionic and covalent compounds? 11. Circle all of the elements in the list below that CAN form double bonds. H Br C N O Cl 12. How do you determine which atom should be the central atom in a Lewis structure? 13. Circle the words that correctly complete the statement: Breaking a chemical bond requires/releases energy, while forming a chemical bond requires/releases energy. 14. Which intermolecular force(s) is/are present in the molecules below: a. I2 b. c. Choose at least 3 of the problems from the chart to solve. O3 MUST be one that you choose. Lewis Structure Formula VSEPR Geometry Polar or NonPolar NH4 +1 OCN-1 CF2H2 F2O CH2O O3 SF6 14. How many electrons are shared in a single bond? A double bond? A triple bond? 15. Magnesium combines with 2 chlorine atoms. Draw the dot structures, use arrows to show the transfer of electrons, and write the formula for the compound.