Final
advertisement

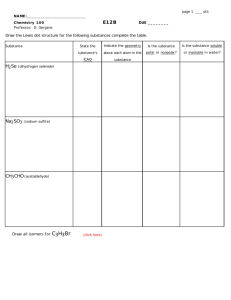
2006 Fluorocarbons Final (250 pts doubled is 500) NAME: 1) Easy Questions (90pts) a) (16pts) Where does the name fluorine come from? What is a fluorocarbon? What is the name of the only known enzyme to create a C-F bond? Name a fluorinated product you encountered this week. Give two similarities between Hydrogen and Fluorine. What are the three characteristics that make fluorine such a special and unique substituent? Name four famous fluorine chemists mentioned this semester (Dr. Roche is not famous!) 533582413 Page 1 b) (22pts) Draw a Lewis structure with lone pairs for a: fluorine atom fluorine molecule fluoride ion hydrogen fluoride State and briefly explain one historical reason why organofluorine chemistry grew rapidly during the 1900’s. Give one possible reason why nature finds it (almost) impossible to synthesize organofluorine compounds? Circle which bond is longer C-H or C-F Circle which bond is more polar C-H or C-F Circle the molecule with the stronger σ bond: CF4 CF2H2 BBr3 BCl3 F-F Cl-Cl 533582413 Page 2 c) (12pts) Match the reagents with the correct process: KF, Sulpholane Nucleophilic Trifluoromethylation (CH3OCH2CH2)2NSF3 Electrophilic Trifluoromethylation CsF, Diglyme Oxidative Fluorination Nucleophilic Epoxidation CoF3 Cartman Chiral Fluoridation t BuOOH, tBuLi Electrophilic Epoxidation F2 Nucleophilic Fluorination O F3C Migratory Insertion O O CF3 O , heat Poptart Epoxidation Reductive Defluorination Garrison Cycloaddition N TfO + F - CF3-TMS, TBAF Electrophilic Fluorination Fellonthefloorification Oxidative Elimination Pb(OAc)4, HF Radical Fluorination SF4, HF Radical Trifluoromethylation F2, HCO2H DeoxoFluorination 533582413 Page 3 d) (20pts) These ten questions are True/False: Selectfluor™ is more reactive and dangerous than elemental Fluorine. BAST and DAST reagents are safer alternatives to SF4. Perfluorocarbons and analogous hydrocarbons have similar boiling points. Hexafluorobenzene will undergo Nucleophilic Aromatic Substitution reactions. Perfluoroalkyl-alkynes are more stable than fluoro-alkynes. Perfluorocarbons are normally more dense than hydrocarbons. Perfluorocarbons will undergo reductive defluorination reactions with strong electron donors. Fluoride ion is a worse nucleophile in methanol than chloroform. Tetrafluoroethene undergoes thermal concerted [2+2] cycloadditions. 19 F is more naturally abundant than 18F. 533582413 Page 4 e) (20 pts) Circle the weakest sigma bond in each molecule: H F C H F H HH F H F H H H F H H H H H H Circle the more stable situation in the following pairs: a) b) F H F F F c) d) e) F H F Cl F H F Rank the following aromatics in increasing reactivity towards nucleophilic aromatic substitution reactions. CH3 NO2 F F OH F 533582413 F Page 5 2) Transformations (4x15 = 60pts) Fill in the products: NaOCl F3C CF3 F3C F CH3ONa O O SF4 / HF H DAST, hexane Heat HO2C 1) KOH 2)CF3-Si(CH3)3, CsF, THF 3) H2O CO2H F2/N2 CoF3 HCO2H SelectFluor CH3CN H-Br Peroxides F2C CFH (two products) CF2=CCl2 Heat 533582413 Page 6 3) Detailed Questions (3x20 = 60pts) Answer three of the four following questions: a) Explain why PFIB is more reactive than HFP is more reactive than TFE towards nucleophiles. less reactivemore reactive CF2=CF2 < CF2=CFCF3 < 533582413 CF2=C(CF3)2 Page 7 b) In this DAST reaction, explain why the three different solvents encourage the three different products. OH hexane F N S F CH3CH2 F CH3CH2 CH2Cl2 THF F F 533582413 Page 8 c) Explain the formation, and the relative ratios, of the three products generated in the following reaction. CF2 CF2 80oC F2C CF2 F2C CF2 (82%) 533582413 (14%) CF2 CF2 (4%) Page 9 d) Why did the Montreal Protocol seek to put a ban on the production and use of chlorofluorocarbons? 533582413 Page 10 4) Mechanisms (2x20 = 40pts) Using curly arrows, write mechanisms for two of the three following reactions. a) CCl3 HF, SbF5 533582413 CF3 Page 11 b) Cl Cl Cl Cl Cl Cl KF, N.M.P. 180oC Cl Cl 533582413 F F F F F F F F Page 12 c) Ph Ph F2, H2SO4 CH3CN, H2O, reflux 533582413 O Ph C CF2-Ph Page 13