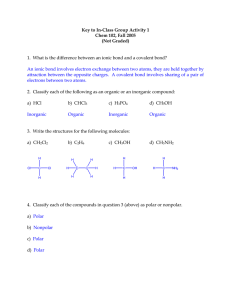
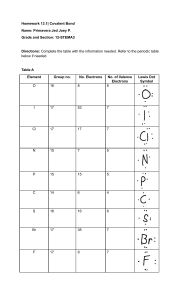
Covalent Bond Practice: 1. What is a covalent bond? 2. What is it between? 3. After two elements share electrons they are called? 4. State if the following would be a single, double or triple bond a. HF b. PAs c. PoTe d. Br2 5. Where on the periodic table do you find the elements that share the most unequally? 6. State if the following are Polar or Non- polar? a. HBr b. Cl2 c. SeF2 d. GeH4 e. AtCl 7. Are bonds stronger between polar or nonpolar molecules? 8. Draw Lewis Structures for the following a. SiH4 b. Cl2 c. PI3 d. S2