Biological Molecules & the Chemistry of Life Key
advertisement

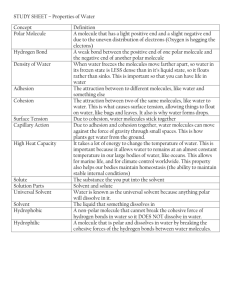
1 Biology 12 Biological Molecules & the Chemistry of Life Key A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. Because it has charged atoms. Since the atoms are different sizes, the O which is larger, holds the electrons in its orbit more frequently than the hydrogen will, therefore giving it a negative charge and the hydrogen a positive charge. 2. What holds a water molecule together? What type of bond holds several water molecules together? Where will this bond form? How would you describe this type of bond? Covalent bonds form between 1 O and 2 H. Hydrogen bonds form between the H+ of one water molecule and the O- of a neighboring molecule thereby holding them together weakly. 3. List 4 functions of water in the body. Give an example of each function. i. Universal solvent and helps in chemical reactions, e.g. digestion of starch into glucose molecules. ii. Helps transport the dissolved and suspended substances since it is cohesive and clings to the surfaces of vessels it will fill blood vessels and move freely through them. e.g. the transport of O2 into blood. iii. Temperature is slow maintains temperature in living systems at a constant level. iv. High heat of vaporization keeps living systems from over heating (water absorbs a lot of heat before it will evaporate so it can be used to remove heat from a system through sweating or panting). Acids and Bases 4. Give the major characteristics of an acid and relate acids to the pH scale. Molecules that dissociate in water and release H+ , they are sour tasting. The greater the dissociation the stronger the acid and so the lower the pH. (pH 1 –pH 7). 2 5. Give the major characteristic of a base and relate bases to the pH scale. Molecules that can take up H+ or release OH-, they are bitter tasting. The stronger the base the greater the dissociation of the molecules the more OH- in solution (less H+) and so the higher on the pH scale it will be. (pH 7 – pH 14) 6. How many more H+ would there be in a solution of pH3 than in a solution of pH8? 100 000 X more, or (10 8-3 ) 105 X more Buffers Hemoglobin in red blood cells acts as a buffer by preventing a drop in pH of the blood. It is a tertiary protein that bonds to iron, which gives it its red hue when carrying oxygen. 7. Which type of ion, H+ or OH-, is hemoglobin capable of loosely bonding with? It must bond to free H+ if it is to prevent a drop in pH since it is the H+ that makes a solution acidic. 8. a. Describe anemia i.e. what is it, what are the symptoms, and how do you become anemic? (do not refer to pernicious anemia) Anemia is a disease caused by low hemoglobin counts. A person with anemia will feel tired, no energy, generally pale (especially membranes of the eye lids). Poor diet (not enough sources of iron in the diet and so not making enough hemoglobin) and excessive bleeding can cause anemia. b. Why would an anemic person be in greater danger of a blood pH drop? An anemic person has less iron therefore less hemoglobin can be made and found in their blood and so there would be less ability to buffer against a rise in H+. B. Some Important Organic Molecules Study Figure 2.17 page 32 carefully. It shows the formation of an organic molecule (dehydration synthesis) 9. Look carefully at the bonds formed during synthesis of a large molecule. Water is produced, where does it come from (be specific)? When two organic monomers are joined one loses a H+ and the other loses an OH- thus creating a water molecule. 10. When the reaction is reversed (hydrolysis) water is needed. What are the 2 roles water molecules play in this reaction? 3 To break or disrupt the C-O bond that was formed (polarity of water enables this) and the “lost H+ and OH-“ are replaced (reformed) from the dissociated ions of water. Proteins 11. Although many proteins are enzymes, there are many other types of proteins in our bodies. Give 4 other types of proteins (HEATS: acronym to remember types) and their role in living things. Hormones, enzymes, antibodies, transporters (hemoglobin, albumin), structural (keratin, collagen, collagen, actin, myosin). 12. Explain the derivation (origin) of the term "amino acid". This molecule has two parts: the amino group (-NH2) and the acid group (-COOH) thus its name amino acid. 13. Draw the structural formula of a typical amino acid. Circle the amino group, acid group and remainder. Label them. Amino group Acid group (carboxyl) 14. Why are there twenty types of R groups? Because there are twenty different amino acids 15. Clearly identify the difference between the amino acid cysteine and the amino acid alanine. Cysteine has an atom of sulphur in its R group that is absent in alanine. 16. Diagram the joining of 2 amino acids together through dehydration synthesis to form a dipeptide with a peptide bond. (Figure 2.25 p. 38 - 11th Edition). Highlight the peptide bond. 4 17. a) When does a polypeptide become a protein? Once it has been released from the ribosome, i.e. the stop codon has been read (primary structure). Or when it takes on its 2, tertiary or quaternary structure. b) Where does this occur in a cell? At the ribosomes in the cytoplasm or RER; or in the ER, and perhaps Golgi apparatus, if it is being secreted. 18. Describe the four structures proteins can assume in terms of what they are made of and the bonds holding them together. Make a sketch (very simple) for each labeling relevant bonds. Once the sequence of amino acids is laid out then hydrogen bonds will form between different polar molecules (certain amino acids) of the polypeptide chain to form the secondary structure. The tertiary structure is formed when there are covalent, ionic and/or hydrogen bonds formed between the R groups of the chain twisting it into a particular 3D shape. The quaternary structure forms when 2 or more chains link together 19. What is meant by protein denaturation and what is its significance in living organisms (specifically enzymes). The change in a protein's secondary, tertiary or quaternary bonds is due to a change in pH or temp. It results in a shape change that makes the protein nonfunctional. Carbohydrates 20. By looking at a structural formula of a molecule, how would you know you were looking at a carbohydrate? It consists of H and O in a 2:1 ratio. 21. How is a monosaccharide converted to a disaccharide? Give examples. What is the empirical formula for glucose? Through a dehydration synthesis reaction bonding 2 monomers together. E.g. glucose + glucose = maltose; or glucose + fructose = sucrose; or glucose + galactose = lactose. The formula for glucose is C6H12O6 5 22. How are monosaccharides converted to polysaccharides? By the repeated dehydration synthesis reactions between monomers (glucose) to make a chain 100’s of monomers long, e.g. starch, cellulose, glycogen. 23. Distinguish between starch and glycogen and sugars (e.g. sucrose). Why is glucose such an important molecule? All are polymers made of glucose molecules, but starch is not as branched as a glycogen macromolecule and sucrose is only 2 monomers long. Starch is a storage form in plants, glycogen in animals while sucrose is quickly digested and forms an immediate energy source. Glucose is important because it can be easily transported from one place to another, is a short-term storage form of chemical energy and can be readily used in the production of ATP, it also is the building block for other carbohydrates. 24. How would you distinguish a cellulose molecule from a starch molecule (2 ways)? Why is cellulose undigestible? Cellulose is an unbranched chain of glucose molecules which are joined by carbon oxygen bonds that reverse their orientation for each monomer added to the chain. Because the bonds joining the O and H are different and can’t be broken by the enzymes we have it is indigestible by us. Only certain monerans (bacteria) have these enzymes and that is why bacteria can be found in mammalian digestive tracts, to help us digest plant food. Lipids 25. Explain how a neutral fat is synthesized. . The union of 1 glycerol molecule with 3 fatty acid molecules. 3 H2O molecules released in the process. 26. Explain how a neutral fat is hydrolyzed. If 3 H2O molecules are added then the glycerol and fatty acids will break apart. 27. Define what saturated and unsaturated fats mean. A C - H bond is considered energy rich. How much more energy per gram do fats contain than carbohydrates? A saturated fat has all C bonds filled with H (no double bonds between C atoms) while an unsaturated fat will still have some double bonds between the C atoms. Fats have 3X the energy than carbs. 28. Read the Health Focus on page 36 and compare trans fats to polyunsaturated fats. 6 Trans fats are oils that have been hydrogenated and therefore are solid at room temperature and are considered saturated. Polyunsaturated fats are oils and will have many double bonds. Phospholipids 29. Differentiate between a lipid and a phospholipid. A phospholipid has one less fatty acid than a lipid and in its place is a phosphate group. This group can ionize and so carries a charge (polar). The 2 fatty acids are non-polar and form the “tails” of the molecule. Lipids in general are nonpolar. 30. Identify one significant use of phospholipids in living organisms. They form the plasma membrane of all cells. Steroids 31. a. What are steroids made of? Steroids are lipids but they have a backbone of 4 carbon rings (the monomers have folded back on themselves to form these rings) with different functional groups attached distinguishes them. The basic steroid is cholesterol all others are made from this. b. Where are they made in the cell? In the body? In the smooth ER… in the body? Adrenal gland, ovaries and testes. c. List 2 examples of steroids. estrogen and testosterone Nucleic Acids Read the pages on DNA, RNA & nucleotides, we will be going into more detail with these polymers in the future but you must know their basic structure and function now. Answer the question on ATP below. 32. Sketch a molecule of ATP and describe its structure. ATP is at first a nucleotide consisting of the base adenine bonded to ribose (adenosine) and a phosphate group. However this nucleotide can go on to form additional high energy bonds with 2 more phosphate groups (triphosphate) 33. What process produces ATP? How many ATP molecules can be made from a single glucose molecule? The phosphate molecules are added on to the nucleotide in the mitochondria of all cells thru the chemical reaction called cellular respiration. Glucose bonds are broken; the energy released is used to 7 bond a phosphate group to the nucleotide (adenosine monophosphate). As the 3rd and final phosphate is added the molecule is complete. The ‘burning’ of 1 glucose molecule will produce 36 ATP molecules 34. Why is ATP the “energy currency” of the cell? The energy released is equal to the amount needed to drive most reactions therefore is more efficient form of chemical energy than glucose. 35. Copy out the ATP cycle as given in Figure 6.3, page 101. Since this is a cycle what does this tell you about this molecule? The ATP molecule is recyclable; it can be used over and over again.