Charles's Law Exercise: Volume & Temperature Relationship
advertisement

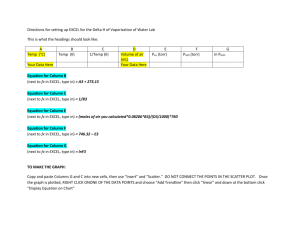
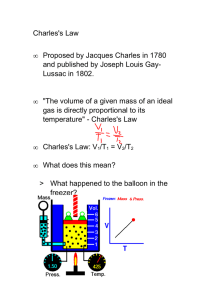
Charles Law Exercise Charles law shows the relationship between volume and temperature. This law states that volume and temperature have a directly proportional relationship. So when you graph volume vs. temperature it should be linear. Procedure: 1. Open an excel workbook and click on file and open from the documents folder the Charles law workbook. 2. In cell A4 enter a value for the number of moles. Keep the number between 1 – 5 moles (It can be a decimal number such as 0.5). 3. In cell A5 enter a pressure in atm. Keep the pressure between 0.5 and 2 atms ( It can be a decimal number such as 1.25 atm). 4. In cell A6 enter a value (You choose it) for the the original temperature in degrees Celsius (This can also be a decimal number.). 5. In cell A7 enter how many degrees Celsius the temperature will change between data points (You choose this number). 6. Highlight your temperature and volume data from cells A9 to B19. 7. Click on the chart wizard icon at the top of the page. Select XY scatter plot. Under chart sub-type select the second one in the left hand column. Then click next. Then click next again to add a title for the graph and labels for the X and Y axis. 8. Click on next then make sure place chart as object in sheet 1 is selected. Then click on Finish. 9. Click on the chart itself. Then go up to the top of the page and click on chart and then click on add trendline. Click on the linear trend / regression type then click on options. 10. Click on forecast backwards and scroll up to 400 units. Then check the display equation on chart and display R2 value on chart options. Then click on ok. 11. Click on file and then click on save as, name the file yournamecharleslawgraphpd(1, 3, or 7). Questions 1. Does this graph illustrate a linear relationship? Why? 2. What does it mean when Jacques Charles stated that volume and temperature have a directly proportional relationship? 3. Change the variables in your equation from X and Y to V and T. Use this equation to determine the temperature when the volume goes to zero (Show this calculation). What is the significance of this temperature? Using the Charles Law Internet Simulation Activity Procedure: 1. Open up Safari and log on to the site www.chem.iastate.edu/group/Greenbowe/sections/projectfolder/flashfiles/gaslawc harles_law.html 2. Click on the show data table button. Move the slider that is just below and watch as the piston adjusts the volume to that particular temperature. 3. Place this new temperature and volume in the data table. Move the slider and collect 9 additional temperatures and volumes. Temperature Volume 4. Open up an excel workbook. At the top of column A write Temperature (K) and at the top of column B write Volume (ml). 5. Transfer the data from your table to columns A and B of the excel workbook. 6. Highlight the data from columns A and B then click on the chart wizard icon in the upper toolbar. 7. Click on XY Scatter Plot and under chart sub-type select the second type in the left hand column. Then click next. 8. Then click next again in order to add a title and labels (including units) for the X and Y axis. Then click next. 9. Make sure place chart as object in sheet 1is selected. Then click on finish. 10. Click on the chart itself. Then go up to the top of the page and click on chart and then click on add trendline. Click on the linear trend / regression type then click on options. 11. Click on forecast backwards and scroll up to 250 units. Then check the display equation on chart and display R2 value on chart options. Then click on ok. 12. Click on file and then click on save as, name the file yournamecharleslawgraphpd(1, 3, or 7). Questions 1. Does this graph demonstrate a linear relationship? How do you know? 2. Using the graph determine the temperature (in degrees K) at which the volume goes to zero. What is the significance of this temperature when it is measured in degrees Kelvin?