Diels-Alder Reaction: Anthracene & Maleic Anhydride Synthesis
The Diels-Alder Reaction of
Anthracene with Maleic Anhydride
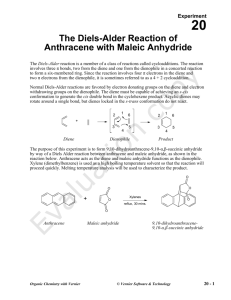
The Diels-Alder reaction is a member of a class of reactions called cycloadditions. The reaction involves three π bonds, two from the diene and one from the dienophile in a concerted reaction to form a six-membered ring. Since the reaction involves four π electrons in the diene and two π electrons from the dienophile, it is sometimes referred to as a 4 + 2 cycloaddition.
Normal Diels-Alder reactions are favored by electron donating groups on the diene and electron withdrawing groups on the dienophile. The diene must be capable of achieving an s-cis conformation to generate the cis double bond in the cyclohexene product. Acyclic dienes may rotate around a single bond, but dienes locked in the s-trans conformation do not react.
1
‡
6
1
2
2 6
+
3 3 5
5
4
4
Diene Dienophile
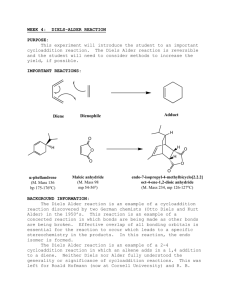
The purpose of this experiment is to form 9,10-dihydroanthracene-9,10-α,β-succinic anhydride by way of a Diels Alder reaction between anthracene and maleic anhydride, as shown in the reaction below. Anthracene acts as the diene and maleic anhydride functions as the dienophile.
Xylene (dimethylbenzene) is used as a high boiling temperature solvent so that the reaction will proceed quickly. Melting temperature analysis will be used to characterize the product.
O
O O
+
O
Xylenes reflux, 30 mins.
O
O
Anthracene Maleic anhydride 9,10-dihydroanthracene-
9,10-α,β-succinic anhydride
OBJECTIVES
In this experiment, you will
Synthesize 9,10-dihydroanthracene-9,10-α,β-succinic anhydride.
Isolate the product.
Measure the melting temperature of your product.
© Vernier Software & Technology 1 Organic Chemistry with Vernier
MATERIALS
Part I Synthesis
50 mL round bottom flask stir bar reflux condenser heating mantle and power controller sand drying tube ring stand with two utility clamps ring stand with filtering flask
Büchner funnel filter paper
10 mL graduated cylinder disposable Pasteur pipets and bulb
100 mL beaker two 250 mL beakers watch glass
Part II Melting Temperature
LabQuest or computer interface
LabQuest App or Logger Pro
Vernier Melt Station glass capillary tubes, one end closed
PROCEDURE
anthracene maleic anhydride xylene
Drierite ® distilled water ice magnetic stir plate balance weighing paper grease spatula cotton plug compressed air
Temperature Probe or thermometer boiling stone (optional) tissues (preferably lint-free) product from Part I mortar and pestle
Part I Synthesis
1. Obtain and wear goggles. Protect your arms and hands by wearing a long-sleeve lab coat and gloves. Conduct this reaction in a fume hood.
2. Weigh out 0.80 g of anthracene and 0.40 g of maleic anhydride and transfer the reagents into a 50 mL round bottom flask containing a stir bar. Record both masses to the nearest 0.01 g.
3. Add 10 mL of xylene to the round bottom flask. CAUTION: Xylene is flammable. Keep away from open flames and hot plates .
4. Set up a reflux with the condenser and a drying tube, making sure to clamp the flask and condenser securely. Remember to grease the joints to prevent the glass from sticking.
Note: Maleic anhydride is water sensitive and the drying tube will help prevent water in the air from entering the flask.
5. Heat the reaction mixture using a heating mantle to reflux (~180°C) for approximately
30 minutes. Monitor the temperature using a Temperature Probe or thermometer.
6. While waiting, prepare two ice water baths using two 250 mL beakers.
7. Obtain approximately 5 mL of xylenes in the 100 mL beaker. Place the beaker in the ice water bath to cool.
2 Organic Chemistry with Vernier
The Diels-Alder Reaction of Anthracene with Maleic Anhydride
8. After 30 minutes, let the reaction flask warm to room temperature. Wait at least 15 minutes for the flask to warm to room temperature. Then, place the flask in the second ice water bath for 10 minutes. You should observe crystallization at this point.
9. Collect the solid. a.
Weigh the filter paper and record the mass to the nearest 0.01 g. b.
Set up a vacuum filtration with the Büchner funnel. c.
Filter the solid and wash the solid with ~5 mL of cold xylene. d.
Place the filter paper containing the solid on a watch glass and gently direct a stream of air
(low flow) to thoroughly dry the solid.
10. Weigh the filter paper and dried product. Record the mass to the nearest 0.01 g. The sample should be completely dried before taking a melting temperature.
Part II Test the Melting Temperature
11. Obtain a small amount of your product. The solid should be in a powdered form. If it is not, use a mortar and pestle to carefully grind the solid to a powder.
12. Prepare a sample for melting. a.
Pack a capillary tube 3–4 mm (~1/8 inch) deep with your sample by inserting the open end into a small pile of the solid. A small amount of the solid will be pushed up into the tube. b.
Tap the closed end of the capillary tube on the table top to compress the sample into the closed end. c.
Check the control knob on the Melt Station to confirm that it is in the Off position. d.
Carefully insert the capillary tube of solid into one of the sample holders of the Melt
Station.
13. Connect the Melt Station power supply to a powered electrical outlet.
14. Connect the Melt Station sensor cable to LabQuest or to a computer interface.
15. Start the data-collection program, then choose New from the File menu. You are now set up to take melting temperature data for up to 20 minutes.
16. In the first trial, you will want to observe the melting process and make a rough estimate of the melting temperature of your sample. Don’t worry if the heating rate is a bit too rapid, and the sample melts too quickly. To do this: a.
Start data collection. b.
On the Melt Station, turn the control knob to a setting of 180ºC. The red light will turn on indicating active heating. c.
Carefully observe your sample. If the solid begins to melt, click Mark to mark the temperature on your graph (or press the D key on the computer or the OK button on
LabQuest). When the entire solid has completely melted, click Mark again. The two values marked on your graph describe the estimated melting temperature range of your substance. d.
If the solid does not melt by the time the temperature gets to 150ºC, turn the control knob to the 220ºC setting. Continue observing your sample, and if the sample begins to melt, mark the temperatures on the graph as previously described.
Organic Chemistry with Vernier 3
e.
If the sample has not melted by the time the temperature gets to 190ºC, turn the knob to the Rapid Heat setting. When the sample finally begins to melt, mark the graph as previously indicated. f.
When you have determined the approximate melting temperature range for the sample, stop data collection. Store the run by tapping the File Cabinet icon in LabQuest, or choosing Store Latest Run from the Experiment menu in Logger Pro . Discard the capillary tube and sample as directed by your instructor. g.
On the Melt Station, turn the control knob to the Fan/Cooling setting to get ready for the next trial. The blue light will turn on indicating that the fan is cooling the Melt Station.
17. Now that you have a rough idea of the melting temperature, a more accurate determination of the melting temperature can be made. Use a previously prepared sample in a capillary tube, as described in Steps 11
12, to determine the melting temperature of the sample: a.
Start data collection. b.
On the Melt Station, turn the control knob to the Rapid Heat setting. c.
Carefully observe the temperature vs . time graph. When the temperature is within approximately 10ºC of the lowest possible melting temperature of your sample, turn the control knob to a temperature setting corresponding to your expected melting temperature. d.
Carefully observe your sample. When the solid begins to melt, click Mark to mark the temperature on your graph. When the entire solid has completely melted, click Mark again. The two values marked on your graph describe the estimated melting temperature range of your substance. When you are finished with this step, stop data collection. e.
Store the run. f.
Discard the capillary tube and sample as directed by your instructor. g.
On the Melt Station, turn the control knob to the Fan/Cooling setting to get ready for the next trial.
18. Repeat Step 17 until you have determined the melting temperature range of the solid.
19. At the end of the experiment, turn the control knob on the Melt Station to Off.
4 Organic Chemistry with Vernier
The Diels-Alder Reaction of Anthracene with Maleic Anhydride
DATA TABLE
Part I Synthesis of 9,10-dihydroanthracene-9,10α,β-succinic anhydride
Mass of anthracene (g)
Mass of maleic anhydride (g)
Mass of filter paper (g)
Mass of filter paper and product (g)
Mass of product (g)
Part II Melting Temperature
Measured melting temperature range (°C)
9,10-dihydroanthracene-9,10-
α,β-succinic anhydride
DATA ANALYSIS
1. What is the theoretical yield of 9,10-dihydroanthracene-9,10-α,β-succinic anhydride in your synthesis? What is the actual yield?
Organic Chemistry with Vernier 5
INSTRUCTOR INFORMATION
1. Xylenes are flammable. Keep away from open flames and hot plates.
2. Assemble the drying tube by placing a small piece of glass wool or cotton near the tip, fill with Drierite
®
, and cover with a second piece of glass wool or cotton.
3. The product will start to precipitate as the reaction flask cools to room temperature.
4. Dry the product completely before taking a melting temperature.
5. The melting temperature range for 9,10-dihydroanthracene-9,10-α,β-succinic anhydride is
261–262°C.
6. Dispose of waste appropriately.
HAZARD ALERTS
Anthracene: May cause respiratory tract irritation. Skin and eye irritant. Moderately toxic by ingestion. HMIS Classification: Health hazard
0, Flammability
1, Physical hazard
0.
Maleic anhydride: Toxic by inhalation and ingestion. Severe eye and skin irritant. HMIS
Classification: Health hazard
3, Flammability
0, Physical hazard
0.
Xylenes: Moderate fire hazard (flash point 25°C). Moderately toxic by inhalation or ingestion.
Causes skin and eye irritation. HMIS Classification: Health hazard
2, Flammability
3, Physical hazard
0.
Drierite
®
: Toxic by ingestion. May cause respiratory tract irritation. Skin and eye irritant. HMIS
Classification: Health hazard
2, Flammability
0, Physical hazard–0.
The hazard information reference is Sigma-Aldrich Co.,
1-800-325-3010, www.sigmaaldrich.com/safety-center/msds-search.html.
COMPOUND INFORMATION
Compound anthracene maleic anhydride
Chemical formula Melting temperature range (
C)
C
14
H
10
210 –215
C
4
H
2
O
3
51 –56
Compound Chemical formula xylenes C
8
H
10
Boiling temperature
range (
C)
137 –140
Molar mass (g/mol)
106.16
Molar mass (g/mol)
178.23
98.06
Density (g/mL) at
25°C
0.86
6 Organic Chemistry with Vernier
SAMPLE RESULTS
The Diels-Alder Reaction of Anthracene with Maleic Anhydride
Melting temperature graph of
9,10-dihydroanthracene-9,10-α,β-succinic anhydride.
Organic Chemistry with Vernier 7