Problem Set #1 - smhs
advertisement
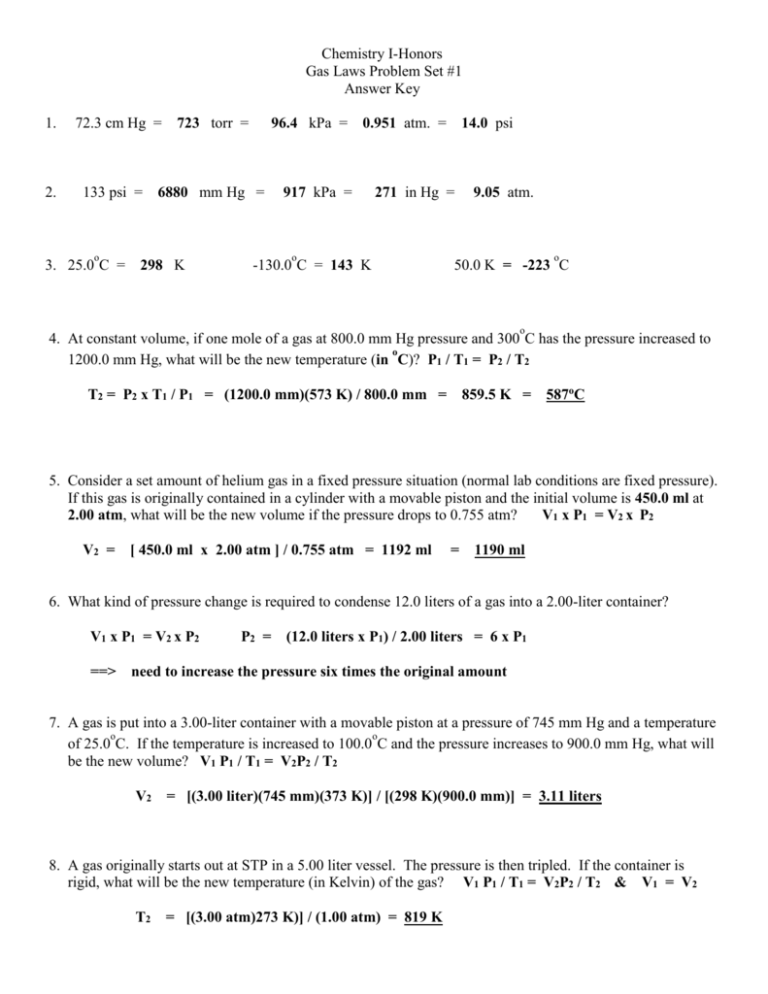
Chemistry I-Honors Gas Laws Problem Set #1 Answer Key 1. 2. 72.3 cm Hg = 723 torr = 96.4 kPa = 0.951 atm. = 14.0 psi 133 psi = 6880 mm Hg = o 3. 25.0 C = 298 K 917 kPa = 271 in Hg = o -130.0 C = 143 K 9.05 atm. o 50.0 K = -223 C o 4. At constant volume, if one mole of a gas at 800.0 mm Hg pressure and 300 C has the pressure increased to o 1200.0 mm Hg, what will be the new temperature (in C)? P1 / T1 = P2 / T2 T2 = P2 x T1 / P1 = (1200.0 mm)(573 K) / 800.0 mm = 859.5 K = 587oC 5. Consider a set amount of helium gas in a fixed pressure situation (normal lab conditions are fixed pressure). If this gas is originally contained in a cylinder with a movable piston and the initial volume is 450.0 ml at 2.00 atm, what will be the new volume if the pressure drops to 0.755 atm? V1 x P1 = V2 x P2 V2 = [ 450.0 ml x 2.00 atm ] / 0.755 atm = 1192 ml = 1190 ml 6. What kind of pressure change is required to condense 12.0 liters of a gas into a 2.00-liter container? V1 x P1 = V2 x P2 ==> P2 = (12.0 liters x P1) / 2.00 liters = 6 x P1 need to increase the pressure six times the original amount 7. A gas is put into a 3.00-liter container with a movable piston at a pressure of 745 mm Hg and a temperature o o of 25.0 C. If the temperature is increased to 100.0 C and the pressure increases to 900.0 mm Hg, what will be the new volume? V1 P1 / T1 = V2P2 / T2 V2 = [(3.00 liter)(745 mm)(373 K)] / [(298 K)(900.0 mm)] = 3.11 liters 8. A gas originally starts out at STP in a 5.00 liter vessel. The pressure is then tripled. If the container is rigid, what will be the new temperature (in Kelvin) of the gas? V1 P1 / T1 = V2P2 / T2 & V1 = V2 T2 = [(3.00 atm)273 K)] / (1.00 atm) = 819 K











