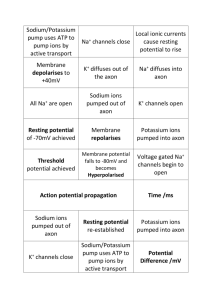
Neuronal Membrane
advertisement

Neuronal membrane at rest
정성권 교수 (schung@med.skku.ac.kr)
Lecture Objectives
1. Basic concepts:
hyperpolarization/depolarization, resting membrane potential
equilibrium (Nernst) potential, Goldman equation
2. Two major reasons for the generation of resting membrane potential.
3. Reversal potential for K+: EK = 61.5 mV log { [K+]o /[K+]i}
4. Calculating the resting membrane potential using Goldman equation.
Vm = 61.5 mV log {Pk[K+]o + PNa[Na+]o} / {Pk[K+] i+ PNa[Na+]i}
Animations for further studies
1.
Mechanism for the generation of resting membrane potential
http://bcs.whfreeman.com/thelifewire/content/chp44/4401s.swf
2.
Molecular structure of K+ ion channel
http://www.youtube.com/watch?v=UqxzSrjzJ70 - The potassium channel structure
3.
Voltage-clamp method
http://www.sumanasinc.com/webcontent/animations/content/voltage_clamp.html
Problems
1. Calculate resting membrane potential if Na+ permeability is 10 times larger than K+ permeability.
ENa = +62 mV, EK = -80 mV
3. What are the molecular mechanisms for the selectivity of Na+, K+ channels towards Na+, K+ ions?
K+ channel, animation #3;
Na+ channel, figure below
4. Once opened, large number of K+ ion (1 x 107) can flow through single K+ channel molecule in one
second!!! Calculate the current level (in pA) through this K+ channel.
elementary charge = 1.6 x 10-19 C (coulombs);
A (ampere) = C / s; pA = 10-12 A
5. In order to generate 100 mV potential across cell membrane, how many K+ ions have to move from the
inside of the cell to the outside? (12 m diameter cell)
The farad is defined as the amount of capacitance for which a potential difference of one volt results in a static charge of
one coulomb. 1 F (패럿)은 1 C(쿨롬)의 전기에 의해서 극판 사이의 전압이 1V(볼트) 변화하는 축전기의 전기용량
cell membrane capacitance, 0.01 pF/m2
charges from 6x1023 molecules, 105 C
cell volume, 10-12 liter
K+ concentration in cytosol, 150 mM
6. A patient is found to be hypokalemic (low levels of K+) and the physician prescribes potassium
supplements. Describe why potassium supplements are important in relation to normal neuron function.
7. Plant toxins like digitalis and ouabain inhibit the action of the Na+/K+ pumps of a neuron, what would
eventually happen to the membrane potential of that neuron?
8. What will happen in the resting membrane potential if there was an increase or decrease of extracellular K+
concentration?
9. What will happen in the resting membrane potential if there was an increase or decrease of extracellular
Na+ concentration?
Learning Issue
1.
Voltage-dependent Na+ and K+ current: HODGKIN and HUXLEY'S experiments
(http://physioweb.med.uvm.edu/cardiacep/EP/handh.htm)
Many of the details of the basis of nerve electrical activity came from a series of experiments in the early
1950's, which led to the awarding of the Nobel prize to the English physiologists A. L. Hodgkin and A. F.
Huxley.
If voltage is the governing factor, one needs a method that allows control of the membrane voltage, so that
it is possible to explore the voltage dependence of membrane properties. The "voltage clamp" is a way of
doing this. Without going into the details, one controls the membrane voltage electronically, and measures
the currents across the membrane produced by ion movements. For example, suppose the membrane
voltage is forced to change in a stepwise
fashion from -70 mV to 0 mV:
The terms inward or negative for current in one
direction and outward or positive for current in
the opposite direction are conventions. An
inward movement of positive ions is defined as
negative or inward current, whereas outward
movement of positive ions produces positive or
outward current. Electrically, inward movement
of negative ions cannot be distinguished from
outward movement of positive ions, so inward
movement of negative ions produces a positive
or outward current.
The current measured is produced by the movement of ions, but it does not tell one directly what types of
ions moved. An outward current could be produced by potassium or sodium moving out of the cell, or by
chloride moving into the cell.
To determine which ions are responsible, one can either change the concentrations of ions surrounding the
membrane, or apply chemicals that interfere with transmembrane movements of specific types of ions.
If one takes away the sodium bathing the axon, and produces the same depolarization as above, the
current is altered:
Now put the sodium back in, and block the movement of potassium by a drug:
By such experiments, Hodgkin and Huxley determined that, when a nerve membrane is depolarized above
a threshold value, there is a transient inward or negative current produced by inward movement of sodium,
followed by a more slowly developing and sustained current in the opposite (outward or positive) direction,
produced by efflux of potassium.
The influx of positive sodium ions depolarizes the cell. This influx is transient. It is followed by an increase
in the efflux of positive potassium ions, and this repolarizes the cell. It is not really essential that the
potassium conductance increase when the cell is depolarized, because the potassium conductance is
already high (the resting cell has a high potassium conductance). A cell in which the potassium
conductance remained constant at the resting value would repolarize once the transient increase of sodium
conductance was over. However, the increased potassium conductance that takes place upon
depolarization hastens repolarization.
The Action Potential
정성권 교수 (schung@med.skku.ac.kr)
Lecture Objectives
1. Basic concepts:
Threshold /after-hyperpolarization/ all-or-none principle
Refractory period (absolute, relative)
Patch-clamp method/ voltage-clamp method
Saltatory conduction
2. Generation & termination of action potential – roles of voltage-dependent K+ and Na+ ion channels
3. Origin of threshold of action potential
4. Refractory period determined the frequency coding of stimulation intensity
5. Factors determining the speed of action potential propagation
6. Myelination increase the speed of AP propagation/ disease associated with demyelination
Animations for further studies
1. Mechanism for the generation of action potential
http://highered.mcgraw-hill.com/sites/0072495855/student_view0/chapter14/animation__the_nerve_impulse.html
2. Propagation of action potential – unidirectional vs. bidirectional
http://msjensen.cehd.umn.edu/1135/Links/Animations/Flash/0014-swf_action_potenti.swf
3. Factors determining the velocity of action potential: Saltatory conduction:
http://www.blackwellpublishing.com/matthews/actionp.html
http://wps.prenhall.com/wps/media/objects/1551/1588888/web_tut/07_02/nav/07_02.html
4. Flow of information through various components of the nervous systems: Reflex Arc
http://www.sumanasinc.com/webcontent/animations/content/reflexarcs2.html
Problems
1. Why do most neurons show a sharp rising phase of action potential?
2. What sets the threshold for action potential?
3. What stops the action potential?
4. At the peak of the action potential, Vm is approximately +50 mV. Assuming normal intracellular and
extracellular K+ concentrations, (1) calculate the driving force (in mV) that acts on K+ ions, and (2)
determine the direction in which K+ ions will flow (i.e., into the cell or out of cell).
5. What electrical properties of the squid giant axon membrane are responsible for the refractory
period?
6. A squid axon, 1 mm in diameter has a resting membrane potential of -75 mV. A microelectrode is
inserted into the axon near the middle. It is used to inject sufficient current to depolarize the axon
membrane at that point to -70 mV. A second (recording) microelectrode is inserted in the axon 1
cm away from the first microelectrode. It reads a membrane voltage of -74.32 mV.
a. What is the space constant, , of the axon (in mm)?
* VX = VO/ ex/ , ** ln (7.3529) = 1.995
b. The axon (horizontally extended) is then submerged in heavy, nonconductive fluorocarbon oil to
half its diameter (see figure below). What is the new space constant?
c. What biological phenomenon resembles the fluorocarbon experiment above?
In what way?
7. You are a neurophysiologist recording action potentials from a squid giant axon. Your girlfriend (or
boyfriend) is due to visit your lab in 10 minutes. She (or he) has indicated that she (or he) wishes
to see “big, big” action potential, overshooting 140 mV above the resting membrane potentials.
What would you do to satisfy and impress her (or him)? Explain your idea.
다발성 경화증 (Multiple Sclerosis; MS)
신경섬유를 싸고 있는 수초층이 점점 파괴되어 신경전달이 일시적으로 중단되거나 잘못 전달된다. 특히
시각·감각·팔다리의 움직임과 관련된 신경전달에 이상이 생긴다. 수초층이 떨어져나간 신경조직 때문에
신경전달이 안 되어 영구적인 마비가 올 수도 있다.
전세계에 걸쳐 발생하나 북반구에서 주로 일어나며 대부분 20~40세 사이에 많이 생긴다. 처음에는 팔다
리나 손발을 쓸 때 떨리거나 힘이 없고 눈이 몽롱해지거나 시력이 떨어지며 감각이 이상해지고 걸음걸이가
불안정하며 어지럽고 물체가 2개로 보이고 소변을 못 참는 등의 증상이 일시적으로 나타난다.
이런 초
기증상은 곧 없어지나 몇 달이나 몇 년 뒤에 다시 재발하며 재발할수록 점점 더 심해지고 몇몇 증상은 사라
지지 않고 계속된다. 마침내는 운동신경 장애로 완전한 마비를 일으킨다. 증상이 나타난 뒤 평균생존기간은
25년 정도이고 완전히 못 움직이게 된 뒤로도 5년 정도를 산다. 어떤 경우에는 급성으로 진행되어 몇 달만
에 급격히 나빠지기도 한다.
원인이 확실히 밝혀지지 않아서 확실한 치료법은 없고 증상을 완화시키기 위해 부신피질 호르몬제를 쓴
다. 재발된 뒤에는 한동안 쉬며 과로하지 않고 정신적 스트레스를 피하는 것이 좋다. 면역계가 어떤 바이러
스의 침입을 받고 몇 년이 지난 뒤 바이러스 대신 수초층을 잘못 공격하기 때문이라는 연구결과도 있으나
원인이 되는 바이러스를 밝혀내는 데는 실패했다.
http://www.livingpixels.net/ms.html
8. Two persons, each suffering from MS, may experience very different symptoms of the disease.
a) Explain.
b) How does the destruction of myelin block conductance of the nerve impulse in an affected
nerve fiber?
c) Currently, what are the treatments for MS?
Learning Issues
1. The origin of action potential threshold
아래 그림에서 검은선은 resting membrane potential (Er)을 결정하고 있는 K+ current의 전압-전류
관계로 이 K+ current는 membrane potential과 관계없이 항상 열려있는 channel임. 노란선은 voltagedependent Na+ current의 전압-전류 관계를 보여준다. Na+ channel은 membrane potential이 -30 -40
mV 보다 depolarization 되어야 열리기 시작하기 때문에 Na+ channel을 통해 흐를 수 있는 Na+ ion
driving force는 크지만 Na+ current는 흐르지 않는다. 녹색선은 이 두 가지 Na+, K+ 전류의 합을
나타낸다.
1) Resting membrane potential인 -80 mV에서 positive current를 흘려주어 membrane potential이 -40
mV가 되었을 때, K+ channel을 통한 세포 외부쪽으로의 K+ current 때문에 membrane potential은
negative voltage로 되돌아 올 것이다 (sub-threshold for action potential).
2) Resting membrane potential인 -80 mV에서 positive current를 흘려주어 membrane potential이 -10
mV가 되었을 때, voltage-dependent Na+ channel activation에 의해 세포 안으로 들어오는 Na+
ion의 양이 K+ channel을 통한 세포 외부쪽으로의 K+ current를 압도하기 때문에 membrane
potential은 positive voltage로 될 것이다 (threshold for action potential).
3) 일단 membrane potential이 threshold 이상이 되어 일정 이상의 positive voltage가 되면 voltagedependent Na+ channel의 activation이 이루어져서 더 많은 수의 Na+ channel이 열리고 이에 의해
소위 “positive feed-back” 현상으로 매우 빠른 속도로 membrane potential의 depolarization이
이루어진다.
Depolarization
Inward current
Na channel open
2. Refractory period and Na+ channel inactivation
After opening, Na+ channels spontaneously and rapidly enter the inactivation state. At the peak of
the action potential, all Na+ channels become inactivated. When Na+ channels are inactivated, they
cannot be immediately opened again (Fig. 3).
Figure 3. Na+ channel activation, inactivation, and recovery from inactivation.
Recovery from inactivation is a time- and voltage-dependent process, and full recovery usually
takes about 3–4 ms. Therefore, it takes about 3–4 ms for all Na+ channels to come out of inactivation
in order to be ready for activation (opening) again. The period from the initiation of the action potential
to immediately after the peak is referred to as the absolute refractory period (ARP) (see Figs. 4, 5).
This is the time during which another stimulus given to the neuron (no matter how strong) will not
lead to a second action potential. Thus, because Na+ channels are inactivated during this time,
additional depolarizing stimuli do not lead to new action potentials. The absolute refractory period
takes about 1-2 ms. After this period, Na+ channels begin to recover from inactivation and if strong
enough stimuli are given to the neuron, it may respond again by generating action potentials.
However, during this time, the stimuli given must be stronger than was originally needed when the
neuron was at rest. This situation will continue until all Na + channels have come out of inactivation.
The period during which a stronger than normal stimulus is needed in order to elicit an action potential
is referred to as the relative refractory period (RRP). During the relative refractory period, since pK
remains above its resting value, continued K+ flow out of the cell would tend to oppose any
depolarization caused by opening of Na+ channels that have recovered from inactivation.
Figure 4. Absolute and relative refractory periods.
Figure 5. Recovery of excitability.
In summary, inactivation of Na+ channels is solely responsible for the absolute refractory period.
Both Na+ channel inactivation and the greater than resting pK value are responsible for the relative
refractory period.
The absolute refractory period is responsible for setting the upper limit on the maximum number
of action potentials that can be generated during any given time period. In other words, the absolute
refractory period determines the maximum frequency of action potentials that can be generated at any
point along the axon plasma membrane. This frequency, in turn, has important physiological
implications for how the nervous system can respond to high-frequency stimuli, and also for the ability
of the nervous system to send high-frequency signals to effector organs when needed.
3. Space constant
Axon fiber를 따라 action potential이 전달될 떄, 전류는 fiber 내부뿐만 아니라 (axial resistance
resistance; rax) 세포 밖으로도 흐른다 (membrane resistance; rm). rm은 세포막에 있는 channel에 의해
결정된다.
total axial resistance
= rax l
total membrane resistance
= rm / l ,
l은 fiber의 가상적 길이
두 resistance 값이 같아지는 거리를 space constant () 라고 하며, 다음의 식으로 주어진다.
rm / rax
2 =
이 값은 E 값이 1/e (대략 37%) 정도로 감소하는 거리이며, 2 의 거리는 E 값이 처음보다 1/10
정도를 보이게 된다. Fiber의 직경이 10 m인 실제 세포의 경우 한 signal이 1mm 정도만 가게
되면, 그 E 값의 변화가 없어지는 정도가 된다. 따라서 이 값을 늘여주어야 할 필요가 생기는데
다음의 방법들이 사용된다.
** Space constant의 증가 방법으로는 첫째, axon의 직경을 크게 한다: 오징어의 giant axon 의 경
우는 직경이 500 m (space constant 5 mm). 둘째, membrane resistance를 크게 한다. 셋째, fiber
바깥쪽을 insulation한다. Myelination이 바로 이것이다.
4. Propagation of action potential in myelinated axon