Word
Physical Science: Survey of Student Knowledge – Form B
Set 1: Physical and Chemical Reactions
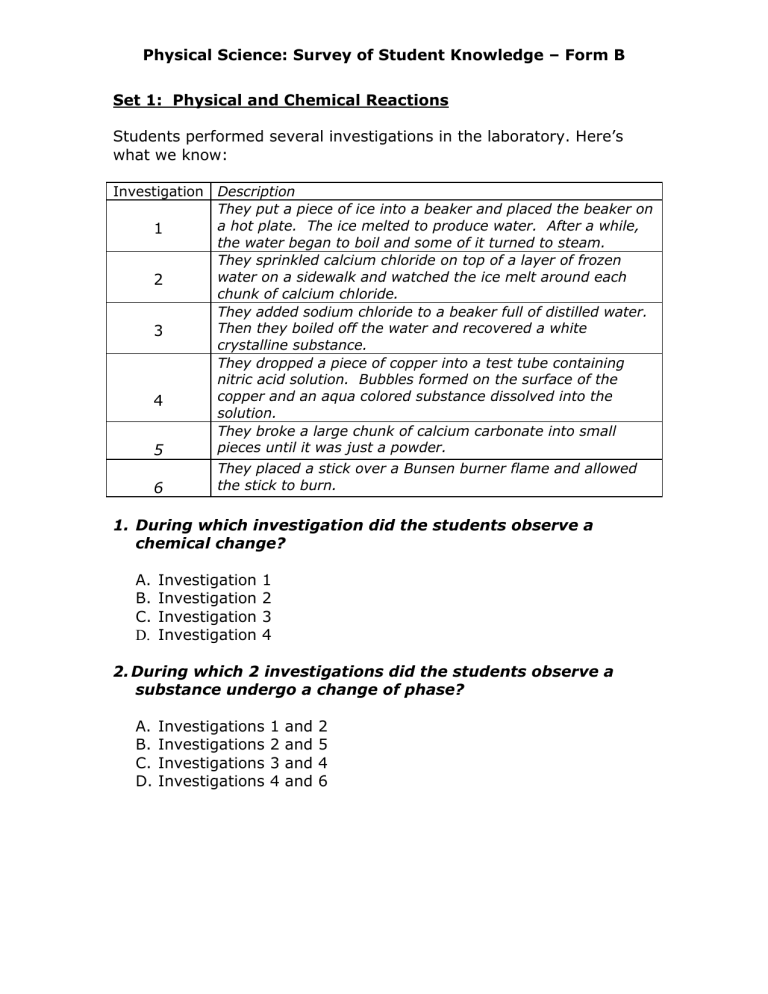
Students performed several investigations in the laboratory. Here’s what we know:
Investigation Description
1
2
3
4
They put a piece of ice into a beaker and placed the beaker on a hot plate. The ice melted to produce water. After a while, the water began to boil and some of it turned to steam.
They sprinkled calcium chloride on top of a layer of frozen water on a sidewalk and watched the ice melt around each chunk of calcium chloride.
They added sodium chloride to a beaker full of distilled water.
Then they boiled off the water and recovered a white crystalline substance.
They dropped a piece of copper into a test tube containing nitric acid solution. Bubbles formed on the surface of the copper and an aqua colored substance dissolved into the solution.
5
6
They broke a large chunk of calcium carbonate into small pieces until it was just a powder.
They placed a stick over a Bunsen burner flame and allowed the stick to burn.
1.
During which investigation did the students observe a chemical change?
A.
Investigation 1
B.
Investigation 2
C.
Investigation 3
D.
Investigation 4
2.
During which 2 investigations did the students observe a substance undergo a change of phase?
A.
Investigations 1 and 2
B.
Investigations 2 and 5
C.
Investigations 3 and 4
D.
Investigations 4 and 6
Physical Science: Survey of Student Knowledge – Form B
3.
Which of these observations is a key characteristic of a
chemical reaction?
A.
A substance that is solid is converted into a substance that is either liquid or gas.
B.
A substance with one set of properties is converted into a substance with different properties.
C.
A substance that has a particular shape or size is converted into a substance with a different shape or size.
D.
A substance that is changed through a chemical reaction cannot be converted back into its original form.
4.
Suppose students start with a mixture of 50% ethanol and
50% water. They heat the mixture at increasingly higher temperatures until most of the ethanol evaporates. The remaining mixture is 95% water. Is this an example of a
chemical reaction?
A.
Yes, because most of the ethanol is driven off, changing the composition of the mixture.
B.
Yes, because the boiling point of the original mixture is lower than the boiling point of the final mixture.
C.
No, because a small portion of the ethanol and water join together to form larger molecules that cannot evaporate.
D.
No, because the chemical structures of ethanol and water remain unchanged.
Physical Science: Survey of Student Knowledge – Form B
Set 2: In Hot Water
Students performed an investigation. Here’s what we know:
Students obtained two identical ceramic mugs.
They filled Mug A with steaming hot water
They filled Mug B with ice-
Mug A cold water from which all the ice was removed.
They placed the mugs at opposite ends of a table.
Every 5 minutes, they measured the temperature of water in each mug and recorded their results in a Mug B graph (Figure 1).
Figure 1 Temperature of water
in Mugs A and B
1.
Suppose that, at 10 minutes, the students combined equal amounts of water from Mugs A and B. What would be the
approximate final temperature of the mixture?
A.
50 0 F because the cold water would absorb most of the heat
B.
80 0 F because that is halfway between the temperatures of water in Mugs A and B when they reach equilibrium
C.
100 0 F because that is halfway between the temperatures of water in Mugs A and B at 10 minutes
D.
140 0 F because the hot water would absorb most of the cold
2.
Which of these is the best explanation for why the
temperature decreased in Mug A?
A.
Cold entered Mug A from the room.
B.
Cold water from Mug B absorbed the heat from Mug A.
C.
Heat from Mug A was absorbed by air in the room.
D.
Heat from Mug A was converted into potential energy.
Physical Science: Survey of Student Knowledge – Form B
3.
How would the results for Mug B be different if students had
left ice cubes floating in the water?
A.
The temperature would drop below the freezing point until the last piece of ice had melted, and then would increase as shown in Figure 1.
B.
The temperature would stay the same until the last piece of ice melted, and then would increase as shown in Figure 1.
C.
The temperature would stay the same until the last piece of ice melted, and then would increase more slowly than that shown in
Figure 1.
D.
The temperature would immediately begin to increase, but would rise more slowly than that shown in Figure 1.
4.
The initial rate of temperature change of water in Mug A was greater than that for water in Mug B. Which of these is the
best explanation for this observation?
A.
Heat enters cool areas more quickly than it leaves warm areas.
B.
The air absorbs heat from warmer objects more quickly than it absorbs heat from cooler objects.
C.
The rate of heat transfer increases with the magnitude of the temperature difference between two objects.
D.
It is a natural for the temperature of objects to decrease as heat moves into the environment.
What is your grade?
1
Grade 5
2
Grade 6
3
Grade 7
4
Grade 8
5
Grade 9
What is your gender?
1
Female
2
Male