Candidacy for VitalStim Therapy®

Candidacy for VitalStim® Therapy
What is VitalStim® Therapy?
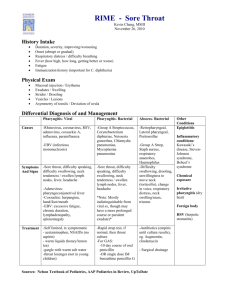
FDA cleared method of rehabilitating swallowing musculature using neuromuscular electrical stimulation (NMES) with concurrent exercise therapy. The therapy is applied to patients suffering from dysphagia (swallowing dysfunction) with signs and symptoms of oropharyngeal muscle weakness.
What is NMES?
Small electrical pulses are delivered to impaired muscle groups through surface electrodes applied over the neck. The pulses induce muscle contractions leading to increased strength and speed of contraction and improved endurance.
Indications for VitalStim® Therapy
Dysphagic patients with signs and symptoms of oropharyngeal neuromuscular impairment are indicated for receiving VitalStim® Therapy. Patients usually present with signs of aspiration, signs of muscle weakness and difficulty managing a normal diet.
Typical patient categories include:
Stroke
Deconditioning as a result of age or comorbidity
Head and neck cancer post radiation and/or surgery
Various other neuromuscular disease processes (e.g., Parkinson’s ALS, etc.)
A patient is indicated for dysphagia therapy when they show signs of, or are at risk for aspiration and/or when they have difficulty managing their diet . One or more of the following signs and symptoms will be evident:
Coughing/clearing of throat after swallow
Abnormal volitional cough
Decreased voice quality (wet, hoarse, weak)
Recurring chest infections
Requires multiple swallows or special maneuvers to clear throat
Difficulty completing a meal
Feeling of food being stuck in the throat
Requires diet to be modified (e.g., thickening, pureed food, soft solids)
Difficulty initiating a swallow
Spillage of food/liquid from lips and/or drooling
Contraindications for receiving VitalStim® Therapy
Patients with active neoplasm or infection in the neck should receive prior clearance by the treating physician before initiating VitalStim® Therapy.
Patients with implanted devices such as pacemakers may receive VitalStim® Therapy.