M.Phil CHEMISTRY08
advertisement

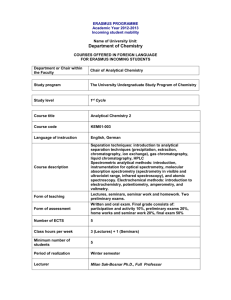
Semester – I Core – I 08MPCH01 RESEARCH METHODOLOGY UNIT – I 1. Research Methodology and Laboratory Techniques 1.1 Survey of Literature: Primary sources – Journals – Periodicals – Patents letters, Communications, notes – Secondary Sources – Titles – reviews, annual reviews – treatises – Monographs, Text Books, Encyclopedia, Beilstein, buyers guide – Abstracts – Subject Index, Substance Index – Author Index, formula index – Index of Journal Article – Materials on a given topic – Information about Specific Compound - Chemical Abstract search through loaded CDS – Aids od Computer in Literature Survey. 1.2 Principles of Research Observation Inference – Hypothesis Generation Testing of hypothesis evaluation of rules, theories and modification of theories and rules. Selection of topics – Instrumental Facility. 1.3 Internet: Introduction to Internet – web browsers – World Wide Web – Search Engines – Literature Survey in Chemistry – Popular Websites in Chemistry – Data base in Chemistry. UNIT – II 2. Documentation Techniques: 2.1 Types of Report – Title – Abstract – Style and Conventions in Writing, Selection of Topic. 2.2 Rough Drafting of the Article – Title, Abstract, Introduction – Literature Review problem and time Limitations, Experimental Methods, results and Discussion, Foot notes, Figures, Data Presentation, Tables, Sign Conventions followed – Conclusions and Recommendations – Bibliography. 2.3 The General Format – Page and Chapter Format use of quotations foot note – tables and figures – Applicability of the findings to common usage – referencing – Abbreviations used. 2.4 Document Preparation using Computers – Use of Microsoft Word, Word Perfect and other Packages for document preparation and formatting – knowledge about TXT, DOC, PDF, PS, HTML formats. UNIT - III 3. Data Analysis: 3.1 True value - standard value - observed value – Error – Types of Errors – Accuracy – Precision, Error Analysis, Minimization of Errors, Deviation from Accurate Results - the Binomial Distribution – the Gaussian Distribution – Mean - - Median – Deviation – from Mean and Median – student’s t-test, Ftest – Significant figures in multiplication – Division – Addition and Subtraction – Curve Fitting method of Least Squares – Linear Regression – Multiple Linear Regression – Slope – Intercept and Correlation Coefficient. UNIT – IV 4. C Programming 4.1 C-fundaments – Identifiers and Keywords – datatypes – constants and variables – symbolic constants – operators and expressions. 4.2 Control Statements: If – else, while, do-while, for, goto, switch…case, break and continue statements. 4.3 Arrays and Pointers – functions – call by value and call by reference – Recursion – Structure and Union – File handling techniques – Library Functions – Basic Structure of C – Programming. UNIT – V 5. C++ Programming 5.1 Basic concepts of OOPs – objects, classes, Data abstraction and encapsulation, inheritance, polymorphism, dynamic binding, message passing – applications of OOPs. 5.2 Classes and structure – Declaration, reference arguments, by value – constructors and destructors – virtual functions – inheritance (simple example only), Overloading – functional overloading, operators overloading (simple example only) – file handling techniques – iostream file. 5.3 Examples of program to draw a circle, hexagon and sphere. References: 1. J. Anderson, B.H. Durston and M.Poole, “Thesis and Assignment Writing”, John Wiley, Sydney 1970. 2. R.Berry, “How to Write a Research Paper”, Pergoman, 1969. 3. Ralph Berry, “The Research Project: How to Write It”, 4th Ed., 4. W.G. Campbell – “Form and Style in thesis Writing”, (1970), Boston M.A; Houghton Mifflin Co., 5. Jerry March, “Advanced Organic Chemistry: Reactions, Mechanisms and Structure”, (1996), 5th Ed., Wiley, 6. James B. Hendrickson, Donald J. Cram George S. Hammond – “Organic Chemistry”, (1970), 3rd Ed., McGraw-Hill Book Co., New York. 4. E. Balagurusamy, “Programming in ANCI C” – Tata McGraw-Hill Publishing Company Limited, New Delhi, 2005. 5. E. Balagurusamy, “Object Oriented Programming with C++” – Tata McGrawHill Publishing Company Limited, New Delhi, 2003. 6. D. Ravichandran, “Programming with C++”, Tata McGraw-Hill Publishing Company Limited, New Delhi, 2005. 7. K.V. Raman, “Computers in Chemistry”, Tata McGraw-Hill Publishing Company Limited, New Delhi, 1993. Semester – I Core – II 08MPCH02 ADVANCED ORGANIC CHEMISRY UNIT – I Nomenclature: Nomenclature of alicyclic compounds – Monocyclic, Bicyclic and tricyclic, Heterocyclics – Compounds containing three atoms viz., Oxygen, Sulphur and Nitrogen (three membered ring to six membered ring). UNIT – II Synthetic Organic Reagents and Reactions: Modern synthetic reactions – Metal hydride reductions of aldehydes and ketones (LiAlH4, NaAlH4, LiBH4, NaBH4). Oxidation – oxidation of alcohols, aldehydes carbon-carbon double bonds by Chromium compounds, Manganese compounds, Peracids and Peroxides. Halogenation – Halogenation of carbonyl compounds, Benzylic hydrogen bonds, Acylation – acylation of active methylene compounds (Basic and acetic conditions), Acylation of enamines. UNIT - III Retro Synthesis: Definition – TM – Retro Synthetic Analysis, FGI Synthesis, Synthetic Equivalence, Disconnection – C–C bond – 1,1, 1,2, 1,3 one group, two group – Equivalent. – Retro Synthesis of Aliphatic Compounds – F.C. Reaction, Wittig Reaction, Dields Alder Reaction, Claisen Condensation, Michal Addition (1,4), Aldol Condensation. UNIT – IV Phase Transfer Catalysts: Introduction – Definition – Mechanism of PTC Reaction – Types of Phase Transfer Catalysts – Advances of Phase Transfer Catalysts. Types of Phase Transfer Reactions – Preparation of Phase Transfer Catalysts – Quarternary salts, Macrocyclic ethers as PTC Tetrahexyl Ammonium Bromide – Benzyl Triethyl Ammonium Chloride – Applications of PTC in Organic Synthesis – Alcohols from alkyl halides – Esterifications – Benzoin condensation – Darzen’s reaction – Oxidation – Reduction. UNIT – V ORD and CD: Circular bireferenges – Circular Dichroism – Cotton effect. Types of cotton effect curves – Axial haloketone rule – statement and its applications. Determination of absolute configuration of ketones. Octant rule and its explanation. Application of octant rule on conformation and configuration of organic and inorganic complexes. References: 1. R. S. Cahn and O. C. Dermer, “Indroduction to Chemical Nomenclature” 5th Edn., Butterworths, London – Boston. 2. “Defenetive Rules for Nomenclature of Organic Chemistry”. Taken from the IUPAC nomenclature of Organic Chemistry 1979 Edn., Published by Pergamon Press, Inc., New York - 10523 USA. 3. Herbert O. House “Modern synthetic reactions” – 2nd Edn., W. A. Benjamin INC., London – 1972. 4. V.K. Ahhwalia – “Organic Synthesis – Special Techniques”, Renn Aggarwal – Narosa Publishing House. 5. E.L. Eliel, stereochemistry of carbon compounds Mc Graw Hill. (1975) 6. I.L. Finar, Organic chemistry vol-II, 5th Ed. ELBS (1975) Semester – I Core – III 08MPCH03 ADVANCED APPLIED CHEMISTRY UNIT – I 1. Chromatography – I 1.1 HPLC: Introduction – Column Packing Materials – Solvent – Detectors – Recorder – Terms and Definitions used in HPLC analysis and applications. 1.2 Gas Chromatography: Introduction – Retention Time – Retention Volume – Efficiency – Career Gases – Preparation of Columns – Solid Supports – Stationary Phases Detectors – Temperature Effect – Quantitative and Qualitative analysis and applications. UNIT – II 2. Chromatography – II 2.1 Gel Permeation Chromatography: (GPC) Introduction – Types of gels – Selection of gels – Gel Preparation – Drying of gels – Packing of the Column Application of the sample – Resolution – Detectors and Applications. 2.2 Gas Chromatography Mass Spectrometry: (GCMS) Introduction – Separators – Carrier gas – Sample Injection – Analyzer and Applications. 2.3 Liquid Chromatography Mass Spectrometry: (LCMS) Introduction – Ionization – Belt Interface – Instrumentation and Applications. UNIT – III 3.1 Nuclear Quadrupole Resonance Spectroscopy: Introduction – Theory of NQR, NQR Spectra and Magnetic Field – Instrumentation – Structural Information from NQR Spectra – Application of NQR. 3.2 Photoelectron Spectroscopy: Introduction – Types of Electron Spectroscopy – Theory of Electron Spectroscopy - Auger Emission Spectroscopy – Instruments for Electron Spectroscopy – Applications of Auger Electron Spectroscopy - Electron Spectroscopy of Chemical Analysis – Chemical Shifts in ESCA – Core Binding Energy – Vibrational Coarse Structure of Electronic Spectra – Applications of ESCA. UNIT – IV 4. Diffraction Studies: 4.1 X – Ray Diffraction: X – rays and their generation; Crystals and the diffraction of X-rays – Laue equations, Bragg’s Law – X-ray diffraction experiment – Powder Method, Single Crystals methods – Principles and Uses. Intensities – R-factors and Structure Determination. High Temperature Powder diffraction. Effect of crystal size on powder pattern – Particle size measurement. 4.2 Neutron Diffraction – Location of light atoms in the presence of heavier ones, distinction between atoms with similar atomic numbers, transition in magnetic crystals. 4.3 Electron Diffraction – by gases, by crystals UNIT – V 5. Desalination of Water: Sea water as a source of drinking water – Desalting – Electro dialysis method, reverse osmosis method, Industrial water treatment – removal of iron, silica, dissolved oxygen, water for laundry work – water for boiler use – removal of slime and algae from water. Deaeration and Deoxygenation of water, chemical deoxygenation of water, clarification of water – coagulation of water Flocculators. References: 1. H. Kaur – “Spectroscopy”, (2005), Pragati Prakashan, Meerut,. 2. Colin. N. Banwell and Elaine M.McCash – “Fundamentals of Molecular Spectroscopy”, 4th Ed., Tata McGraw-Hill Publishing Company Ltd.- New Delhi, 2000. Jag Mohan – “Organic Spectroscopy – Principles and Applications”, (2007), 3. 2nd Ed., Narosa Publishing House. 4. P.S. Kalsi – “Spectroscopy of Organic Compounds”, (2004), 6th Ed., New Age International Publishers. 5. Robert Brawn – “Introduction to Chemical Analysis”, McGraw-Hill Book Company. 6. Christian and James – “Instrumental Analysis”, 2nd Ed. 7. Srivastava, V.K. Srivastava – “Introduction to Chromatography”, S.Chand & Company. 8. Jag Mohan – “Organic Spectroscopy Principles and Applications”, 2nd Ed., Narosa Publishing House. 9. A.F.Wells – “Structural Inorganic Chemistry”, (1962), 3rd Ed., Oxford University Press, Antony R.West, John – “Solid State Chemistry and its Applications”, Wiley & Sons, New York. 10. 11. B.K. Sharma, H.Kaur – “An Introduction to Environmental Pollution”, Goel Publishing House. Semester – I Core – IV 08MPCH 04 MODERN APPROACH TO CHEMISTRY UNIT – I 1. Bio molecules 1.1 Protein Biologically important protein, sources of protein – Biological functions of protein – peptide bond formation – poly peptide chains – classification of protein – properties of protein – Tests for protein 1.2 Nucleic acid Nucleic acid –l occurrence – General structure of Nuclei acid – Types of Nucleic acid – Nucleotide – Nucleosides – Functions of DNA and RNA – comparison of DNA and RNA. Biological functions of Nucleic acid. 1.3 Lipids Introduction, occurrence, sources fat and Atherosclerosis, Fat deposits, Brownfat, component of lipids. Glycerol – structure of lipids – structure of fatty acids – classification of fatty acids. Classifications of lipids – simple lipids – oil – wax – phospholipids glycolipids and derived lipids. UNIT – II 2.1 Methods in Microbiology Methods of sterilization, culture media, pure culture methods, staining of bacteria, counting cells. 2.2 Bacterial diseases of man Airborne diseases, food borne, water borne diseases, and soil borne, sexuallytransmitted and contact diseases – control of micro organism – physical and chemical control – chemotherapeutic agents. Industrial production of penicillin, streptomycin and cephalosporin. UNIT – III 3. Polution: 3.1 Air Pulution:Introduction - Mechanism of distribution – primary, secondary and tertiary dispersion, Essential and non-essential Air pollution & Control Introduction – classification of air pollutants – air pollutants and their effects – Acid rain – photochemical smog – particulates – characteristics and biochemical effects of some air pollutants and their effects – Air pollutants from industrial and other sources. Air pollution control. 3.2 Trace Elements – Pollution and Control Trace elements, physiological role of trace metals, trace elements in marine environment, heavy metals, Industrial uses and pollution sources, Environmental levels, Ecological effects, Bio-chemical effects, Toxicology, environmental fate, control and treatment of the following trace element of the following trace elements: Hg, Cd, Pb, Cr, As, Ni, F, Co and Mn. UNIT – IV 4. Mathematics for Chemists: Functions – constant and variables function – classification of functions – single and many valued function, Explicit and Implicit functions – odd and even functions – inverse functions – Differentiation – Definition – Product Rule – Functions of Function rule – Integration – Definite integral – Methods of Integration – substitution – Integration by parts – Properties of definite integrates – vector and scalar products, inner product and cross product. UNIT – V 5. Human Resource Management: Definition, objectives of Human Resources, Human Resource Policies, Human Resource Planning, Recruitment and Selection, Placement, Promotion, Training and Development. On the job Training, Off the job Training and Development. Performance Evaluation, Wage and Salary Administration, Rewards and Labour Welfare. References: 1. P.D. Sharma, -“Microbiology”, (2000) Rastogi Publications 2nd edn., Meerut. 2. M.J. Pelezar – “Microbiology”, (2004) Tata Mcgraw Hill publications, New Delhi. 3. A. Mani, Dr. A.M. Selvaraj, Dr. L.M. Narayanan, Dr. N. Arumugam –(2004) “Saras publication” Kanyakumari. 4. Dulsy Fatima, R.P. Meyaan pillai, N. Arumugam – (1994) Saras Publication Ist ed. Kanyakumari. 5. B.K. Sharma, H. Kaur –Environmental chemistry 3rd ed. (1996-97) Goel publishing house, Meerut. 6. S.S. Dara – “A Textbook of Environmental chemistry and pollution control Ist ed. S.Chand & Co. Ltd. 7. J.L. Jain – (2001) “Fundamentals of biochemistry” 5th ed., Sulthan chand, New Delhi. 8. A.L. Lehninger and D.L. Nelson – “principle of biochemistry” 2nd ed. CBS publication. 9. M.L. Chatterjee – “A Textbook of Medical and Biochemistry” Vth ed. Jayplee brothers medical publishers pvt. Ltd., New Delhi. 10. Bhupendra Singh – “Mathematics for Chemists”, Pragati Edition. 11 S. Narayanan, T.K. Manicavachagam Pillay – “Calculus”, Vol – I, II, III, S. Viswanathan (Printers & Publishers). 12. Edwin Flippo – “Principles of Personnel Management”, Tata McGraw Hill 13. .G. Nair Latha Nair – “Personnel Management and Industrial Relations”, Chand & Co. 14. Dr.P. Subba Rao – “Human Resource Management” Himalaya Publishing House.