AP Chemistry Practice Final Exam - Multiple Choice Questions
advertisement
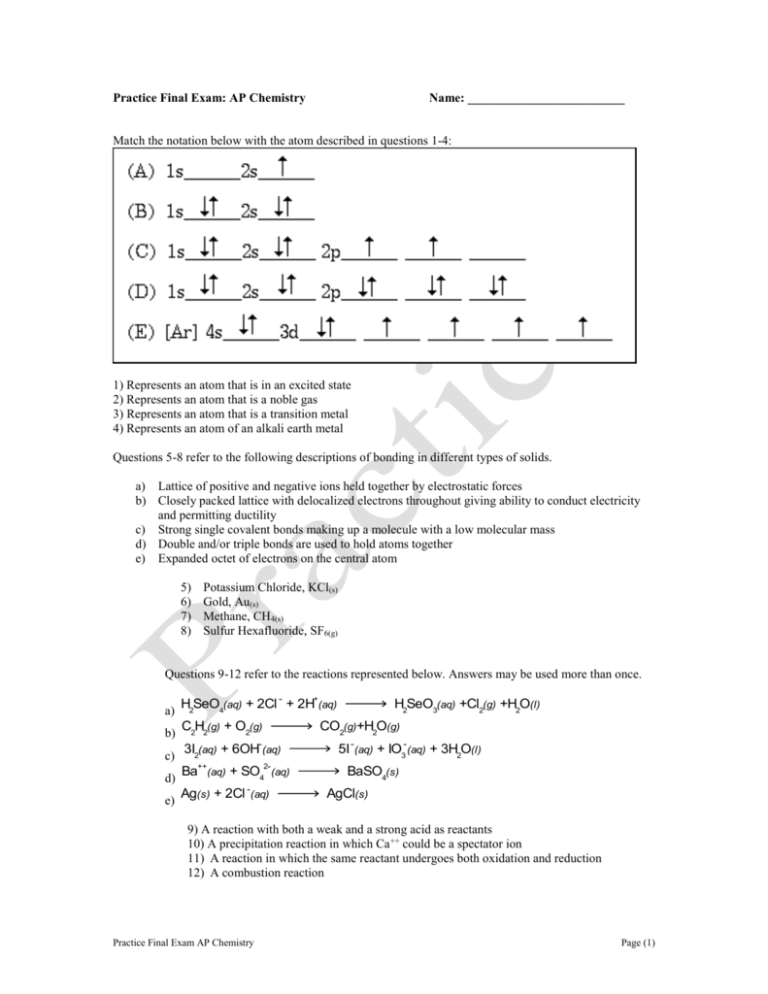
Practice Final Exam: AP Chemistry Name: _________________________ Match the notation below with the atom described in questions 1-4: 1) Represents an atom that is in an excited state 2) Represents an atom that is a noble gas 3) Represents an atom that is a transition metal 4) Represents an atom of an alkali earth metal Questions 5-8 refer to the following descriptions of bonding in different types of solids. a) Lattice of positive and negative ions held together by electrostatic forces b) Closely packed lattice with delocalized electrons throughout giving ability to conduct electricity and permitting ductility c) Strong single covalent bonds making up a molecule with a low molecular mass d) Double and/or triple bonds are used to hold atoms together e) Expanded octet of electrons on the central atom 5) 6) 7) 8) Potassium Chloride, KCl(s) Gold, Au(s) Methane, CH4(s) Sulfur Hexafluoride, SF6(g) Questions 9-12 refer to the reactions represented below. Answers may be used more than once. -- + a) H2SeO4(aq) + 2Cl + 2H (aq) b) C2H2(g) + O2(g) CO2(g)+H2O(g) -- c) 3I2(aq) + 6OH (aq) ++ d) e) Ba (aq) 2-- + SO4 Ag(s) + 2Cl -- H2SeO3(aq) +Cl2(g) +H2O(l) (aq) (aq) 5I -- (aq) -- + IO3 (aq) + 3H2O(l) BaSO4(s) AgCl(s) 9) A reaction with both a weak and a strong acid as reactants 10) A precipitation reaction in which Ca++ could be a spectator ion 11) A reaction in which the same reactant undergoes both oxidation and reduction 12) A combustion reaction Practice Final Exam AP Chemistry Page (1) 13) A 0.10M aqueous solution of potassium phosphate, K3PO4, is a better conductor of electricity than a 0.10M aqueous solution of potassium bromide, KBr. Which of the following best explains this observation? a) K3PO4 is more soluble in water than KBr b) K3PO4 has a higher molar mass than KBr has. c) To prepare a given volume of 0.10M solution, the mass of K3PO4 needed is more than twice the mass of KBr needed. d) More moles of ions are present in a given volume of 0.10M K3PO4 than in the same volume of 0.10M KBr. e) The degree of dissociation of K3PO4 in solution is significantly greater than that of KBr. 14) The element rubidium (atomic mass 85.47) consists of two isotopes, one of mass number 85 (Rb85) and the other of mass number 87 (Rb-87). Which one of the following statements about the isotopes of rubidium is correct? A. Rb-85 is approximately twice as abundant as Rb-87. B. Rb-85 is approximately three times as abundant as Rb-87. C. Rb-87 is approximately twice as abundant as Rb-85. D. Rb-87 is approximately three times as abundant as Rb-85 15) How many protons, neutrons and electrons are present in the ion, 3919K+? a) 39,19,19 b) 20,19,19 c) 19,20,18 d) 19,20,19 e) 19,19,19 16) Which two species contain the same number of electrons? A. B. C. D. 17) Na+ and Ar F- and Na+ Cl+ and Ar Fe3+ and Co2+ If an electron shifts from one energy level in an atom to a lower level, energy is emitted. Which one of the following electron transitions would release the most energy? A. B. C. D. Second level to first level Third level to second level Third level to first level First level to fourth level 18) Which one of the following compounds contains both ionic and covalent bonds? A. NH4Cl (s) B. Na2S(s) C. CO2 (g) D. ClO2 (g) 19) Which one of the following species has a lone pair of electrons on the nitrogen atom? A. NI4+ 20) B. NH4+ C. HCN bonds? D. NO3- All of the following possess a dipole moment except A. HF Practice Final Exam AP Chemistry B. SF2 C. BF3 D. CH2F2 Page (2) 21) An unknown solid melts at high temperature, is insoluble in water, and does not conduct electricity in the solid or liquid states. This substance is most likely to be A. Al B. I2 C. CO2 D. SiO2 E. Ni 22) What molecular geometry (shape) is expected for BH3? A) Linear B) Planar 23) 50 cm3 of 1 M hydrochloric acid 25 cm3 of 5 M sodium chloride 100 cm3 of 0.5 M nitric acid 50 cm3 of 2 M sulphuric acid Which of the molecules given below has/have a nonzero molecular electric dipole? HCl I A. I only 25) D) Tetrahedral E) Octahedral Which of the following solutions contains the largest number of moles of solute? A. B. C. D. 24) C) Pyramidal H2O II NH3 III B. I and II only BF3 IV CH4 V C. IV and V only Use the bond energies below to calculate H for the reaction, H2C=CH2 + H2 H3C—CH3 energy / kJ mol-1 348 612 436 412 Bond C—C C==C H—H C—H A. H = -124 kJ C. H = 48 kJ 26) B. H = -48 kJ D. H = 124 kJ Ignoring heat lost to the surroundings, which of the following is closest to the final temperature of a mixture of water if 100.0 grams of water with a temperature of 40ºC is added to 50.0 g of water at 0.0ºC? A. 30ºC 27) D. I, II, and III only B. 25ºC C. 20ºC D. 15ºC Which of the following processes is/are exothermic? I. H2O(s) H2O(g) II. CO2(g) CO2(s) III. O2(l) O2(g) A. II only Practice Final Exam AP Chemistry B. III only C. I and II only D. I and III only Page (3) 28) In the graph below, what quantity is plotted against the atomic number of the third period elements (sodium to argon)? • • • • • • • • 11 12 13 A. Atomic radius 29) 14 15 16 Atomic number B. Ionisation energy 17 C. Melting point 18 D. Density In which of the following series are the elements arranged in the order of increasing electrical conductivity? A. Mg<Si<S B. Si<S<Mg C. S<Mg<Si D. S<Si<Mg 30) Use the following reaction to assist answering the next three questions 3A + 2B + C 2D + E If there is 1.0 mole of each reactant, which substance is the limiting reagent? a) A b) B c) C d) Both A and B e) Both A and C 31) If 2.0 moles of B react with an excess of A and C, how many moles of products D and E (respectively) are produced? a) 2, 1 b) 3, 2 c) 4, 2 d) 2, 3 e) none of the above are correct choices 32) 3.0 moles of A react with an excess of B and C. If the reaction produces a 50% yield of reactant E, what is the actual yield (in moles) of E? a) 2 moles b) 1.5 moles c) 1.0 moles d) 0.5 moles e) 0.25 moles 33) Look at the reaction below. 2-- 2+ + 3+ 4+ Cr2O7 (aq)+3Sn (aq)+14H (aq) 2Cr (aq)+3Sn (aq)+7H2O(l) Which lists the element oxidized followed by the element reduced? a) Cr, Sn b) O, Sn c) O, Cr d) Cr, H e) Sn, Cr Practice Final Exam AP Chemistry Page (4) 34) Which is closest to the molar concentration of K+ in 0.2M K3PO4? a) 0.30M b) 0.60M c) 1.2M d) 1.6M e) 2.2M 35) Approximately what mass of CuSO4∙5H2O (250 g∙mol-1) is required to prepare 100.0 mL of 0.50M copper (II) sulfate solution? a) 6.125 b) 12.5 c) 25.0 d) 37.5 e) 250 36) A 1.0L solution of 1.0M lithium phosphate, Li3PO4, is diluted to 0.25M. Which is closest to the amount of water _added_ to dilute the solution? a) 1.0L b) 2.0L c) 3.0L d) 4.0L e) 5.0L 37) From the information below, calculate the enthalpy change, ∆Ho, for the reaction: S(s) + O2(g) ---> SO2(g) S(s) + 3/2 O2(g) ---> SO3(g) SO2(g) + 1/2 O2(g) ---> SO3(g) ∆Ho = -395 kJ ∆Ho = -98.3 kJ A. +494 kJ C. -297 kJ 38) B. +297 kJ D. -494 kJ Given the following thermochemical data: N2O4(g) 2NO2(g) ∆Ho = +100 kJ 2NO(g) + O2(g) 2NO2(g) ∆Ho = -50 kJ determine the heat of reaction NO(g) + 1/2O2(g) 1/2N2O4(g) a) -100 b) -75 c) -50 d) -25 e) 0 39) Which two species are isoelectronic, i.e., they contain the same number of electrons? A. Rb+ and K+ B. Cl and Na+ C. Cl- and Br- D. Fe2+ and Co2+ E. N-3 and Na+ 40) Which orbital has a spherical shape? a) 5s b) 4p c) 3d d) 4f e) 5g 41) The atom of which element, in the ground state, has 2 electrons in each of the three p orbitals? a) N b) O c) F d) F+ e) O-1 42) Which set of three quantum numbers (n,l,ml) corresponds to a 2p orbital? a) 2,1,1 b) 2,0,0 c) 2,2,2 d) 2,1,-2 e) 2,0,1 43) An electron in a(n) _____ subshell experiences the greatest effective nuclear charge in a polyelectronic atom. a) 4f b) 4p c) 3d d) 3s e) 4s Practice Final Exam AP Chemistry Page (5) 44) Which are the formal charges respectively of H, C, O in this resonance form of the formate ion: [H-C=O::]-1 A. B. C. D. E. 45) A crystalline solid of formula MX is formed by the reaction of an alkali metal with a halogen. Which of the following conditions will lead to the formation of a crystal with the greatest lattice energy? a) b) c) d) 46) 0,0,0, 0, 0, -1 0,+1,0 0, +1,-1 –2, 0, +1 M+ has a small ionic radius and X- has a small ionic radius M+ has a small ionic radius and X- has a large ionic radius M has a small ionisation energy and X has a large electron affinity M has a small heat of vaporisation and X has a small heat of dissociation Which specie illustrates resonance? a) b) c) d) e) 47) Which molecule is NOT polar? a. H2CO b. CCl c. PBrCl d. SCO 4 48) c. tetrahedral d. square planar e. octahedral What is the maximum amount of copper that could be obtained from a mixture of 5.0 moles of Cu2O and 6.0 moles of C according to the balanced equation below? Cu2O + C 2Cu + CO B. 6.0 moles C. 9.0 moles D. 10.0 moles Which is closest to the volume of 0.20 M sodium hydroxide solution could be made from 20.0 g of solid sodium hydroxide (and an unlimited supply of water)? A. 1.0 dm3 51) 3 2 b. bent A. 5.0 moles 50) e. O What is the shape of the XeCl molecule? a. linear 49) 4 CO32HCN H2CO Br2 NH4+ B. 0.5 dm3 C. 0.25 dm3 D. 2.5 dm3 E. none of these A sample of a pure compound was found to contain only 0.250 g of hydrogen, 1.500 g of carbon, and 8.875g of chlorine. What is the empirical formula of the compound? (H= 1, C = 12, Cl = 35.5) A. CHCl3 Practice Final Exam AP Chemistry B. CH2Cl2 C. CH2Cl D. CH3Cl Page (6)





