CHM 50- Class activity
advertisement

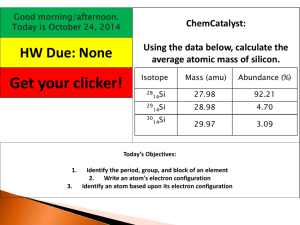
CHM 50 1. Class Activity – Chapter 2 – part 1 Name Two unknown compounds are tested. Compound I contains 15.0 g of hydrogen and 120.0 g of oxygen. Compound II contains 2.0 g of hydrogen and 32.0 g of oxygen. Are the compounds the same? Which law does it follow? 2. Two different compounds are formed by the elements carbon and oxygen. The first compound contains 42.9% by mass carbon and 57.1% by mass oxygen. The second compound contains 27.3% by mass carbon and 72.7% by mass oxygen. Show that the data are consistent with the Law of Multiple Proportions. 3. Label the information provided in the periodic table. 2. What does the atomic number represent? ____________________ or ____________________ 3. What does the atomic mass represent? ____________________ + ____________________ 4. How would you figure the number of protons or electrons in an atom? 5. How would you figure the number of neutrons in an atom? 6. Write isotopic symbols of the form for each of the following isotopes a. Sodium isotope with 12 neutrons b. Dang1 Iodine isotope with 74 neutrons 7. Complete the following table Z A p+ n e- Isotopic symbol 16 17 63 29 74 56 8. Complete the table Isotope Complete the table Isotope Mass (amu) Neon-20 19.992 Neon-21 20.994 Relative Abundance (%) 90.51 Neon-22 9.22 Avg. Atomic Mass = 20.179 Total %: 100.00 9. A new element, Albanesium, has been discovered. 43.2% of all naturally occurring Albanesium has a mass of 292 amu. 46.8% of all Albanesium has a mass of 293 amu. The rest of the Albanesium has a mass of 295 amu. Find the average atomic mass of Albanesium. 10. Explain why atoms have different isotopes. In other words, how is it that helium can exist in three different forms? Dang2 11. Complete the following table Atom or ion Number of protons Number of electrons Electrons lost/gained K+ 12 10 16 2 e- gained 28 12. Convert to moles: a. 12.04 x 1023 atoms He 2e- lost 13. Convert to grams 10.0 moles Na b.1 atom S 5.00 moles Ag c.0.005 grams Cl2 14. Convert to number of atoms: a. 8.50 moles Ca b. 0.001 moles Cd c. 1.0 x 10-5 moles O2 Dang3