There are five C6H14 isomers, shown below as abbreviated line
advertisement

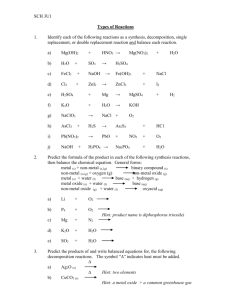
There are five C6H14 isomers, shown below as abbreviated line formulas (A through E): Although these distinct compounds all have the same molecular formula, only one (A) can be called hexane. How then are we to name the others? (A) hexane (B) 2-methylpentane (C)3-methylpentane (D)2,2-dimethylbutane (E)2,3-dimethylbutane Name the following organic compounds according to IUPAC rules. (A)2,4-dimethylpentane (B)2,4-dimethylheptane (C)2,4,6-trimethyloctane (D)3-ethyl-2-methylpetane (E)2,3-dimethylpentane (F)2,2-dimethylhexane (G)2,5-dimethylhexane (H)4-methylheptane (I)3-ethyl-1,1,2,2-tetramethylcyclopentane (J)1,1-dimethylcyclohexane (K)2-ethyl-1,1,3,3-tetramethylcyclopentane (L)2,3-dimethylbutane 1. Draw graphical formulae for; (i) 2-methylpropane (ii) 2,2,6-trimethyloctane 2. Name; (i) CH3C(CH3)2CH2CH3 (ii)CH3CH(CH3)CH2CH(CH3)CH3 2,2-dimethylbutane 2,5-dimethylpentane 3. Draw graphical formulae for; (i) 4-methylhex-1-yne (ii) 3,4-diethylhex-2-ene 4. Name; (i) CH3CH2CH=CH2 (ii) CH3CH2CH(CH3)C≡CCH3 1-butene 4-methylhex-1-yne 5. Draw graphical formulae for; (i) triiodomethane (ii) 1-bromo-3-chloro-4-methylhexane 6. Name; (i) CH3CH2CH2CH2CH2I (ii) CH3CH2CHBrCH2CH3 1-iodopentane 3-bromopentane 7. Draw graphical formulae for; (i) pentan-2-ol (ii) cyclohexane-1,3-diol 8. Name; (i) CH3CH2CH(OH)CH2CH3 (ii) CH3C(CH3)(OH)CH2CH3 3-pentanol 2methyl-butan-2-ol 9. Draw graphical formulae for; (i) cyclohexanone (ii) 3-chlorobutan-2-one 10. Name; (i) CH3CH2CH2CHO (ii) CH3CH(CH3)COCH3 butanal 3-methylbutan-2-one 11. Draw graphical formulae for; (i) 3-aminopropanoic acid (ii) chloroethanoic acid 12. Name; (i) CH3CH2CO2H (ii) CH3CH2CH(OH)CH2COOH propanoic acid 3-hydroxypentanoic acid Summary of Reaction Predictions Note: The following summary is not comprehensive. It is meant to be a simple and concise prediction help. I. Acid Base Reactions A. HX + MOH → _____HM____________ + _____H2O____________ B. Remember: Weak acids and bases must be written in the NIE as _molecules______. II. Precipitation Reactions A. Generally both reactants are salts in solution: AX + BY → ___AY (s)___________ + ______BX(aq)________ (Where AY and/or BX are salts in solution.) B. Net Ionic Equation: A+ + Y- → ___AY(s)______________ III. Hydrolysis Reactions A. A metal oxide reacts with water to form a ___bases (metal hydroxides)__________. B. A nonmetal oxide reacts with water to form an __acids________________. C. A metal hydride reacts with water to form a ___base___________ and __hydrogen gas______. IV. Decomposition Reactions A. Occurs when a single reactant is __breaks up into two or more pieces____. B. Metallic carbonates decompose to form a _metallic oxide___ and _carbon dioxide gas__. C. Metallic hydroxides decompose to form _metallic oxides__ and __water_______. D. Metallic chlorates decompose to form __metallic chlorides__ and __oxygen gas___. E. Metallic sulfates decompose to form __metallic oxides___ and __sulfur trioxide gas____. F. Some acids decompose to form __nonmetal oxides_____ and __water___________. G. Sulfurous acid decomposes in water to form ____sulfur dioxide____ and ____water________. H. Hydrogen peroxide decomposes to form ___water_______ and __hydrogen gas____. V. Ligand Reactions A. Occur between the following metals:__ Fe, Cu, Pb, Ag, Au, Sn, Pt, Co, etc__. and the following ligands: CN-, SCN-, NO2- , NH3, H2O, OH-, halides (F-, Cl-, Br-, I-)_. B. To determine how many ligands to attach to a metal center usually ____double ______ the metal cation’s charge. VI. Redox Reactions A. Metal and nonmetal react to form ___an ionic salt_____. B. When a solid metal replaces the metal anion from a metallic compound it is called a _single replacement reaction____, C. A redox reaction occurs when a halogen replaces a___halide____ salt. D. When MnO4- is placed in an acidic solution with a metal cation, the MnO4- is reduced to ___Mn2+_____________; the metal in solution is oxidized. E. When Cr2O72- is placed in acidic solution with a metal cation, the Cr2O72- is reduced to __Cr3+______________; the metal in solution is oxidized. F. Cu(s) + HNO3(dilute) → __Cu2+__ + ___NO___ + ____H2O____ Name________________________________________________________________________ Reaction Predictions Assignment Write balanced net-ionic (only ions that participate in the reaction are shown) equations below. 1. A solution of potassium phosphate is mixed with a solution of calcium acetate. 2 PO43- + 3 Ca2+ → Ca3(PO4)2 2. A solution of hydrogen peroxide is exposed to strong sunlight. 2 H2O2 → H2O + O2 3. Solid sodium hydride is added to water NaH + H2O → NaOH + H2 4. Propanone is burned in air. C2H6O + 3 O2 → 2 CO2 + 3 H2O 5. A solution of sodium iodide is added to a solution of lead (II) acetate. 2 I- + Pb2+ → PbI2 6. Solid cesium oxide is added to water. Cs2O + H2O → 2 CsOH 7. Solid zinc carbonate is added to 1.0 M sulfuric acid. ZnCO3 + 2 H+ → Zn2+ + CO2 + H2O 8. Solid dinitrogen pentoxide is added to water. N2O5 + H2O → 2 HNO3 9. Magnesium ribbon is burned in oxygen. 2 Mg + O2 → 2 MgO 10. Ammonia gas is mixed with hydrogen chloride gas. NH3 + HCl → NH4Cl 11. Solid sodium acetate is added to 1.0 M hydrobromic acid. NaC2H3O2 + H+ → Na+ HC2H3O2 12. A bar of strontium metal is immersed in a 1.0 M copper (II) nitrate solution. Sr + Cu2+ → Sr2+ + Cu 13. Pure solid phosphorus is burned in air. P4 + 5 O2 → P4O10 14. Sulfur trioxide gas is bubbled into water. SO3 + H2O → H2SO4 15. Excess concentrated potassium hydroxide solution is added to a solution of nickel (II) chloride. Ni2+ + 4 OH- → [Ni(OH)4]216. Excess concentrated aqueous ammonia is added to solid silver chloride. AgCl + NH3 → [Ag(NH3)2]+ + Cl17. Hot hydrogen gas is passed over heated copper (II) oxide solid. H2 + CuO → Cu H2O 18. A 0.02 M hydrochloric acid solution is mixed with an equal volume of a 0.01 M calcium hydroxide solution. H+ + OH- → H2O 19. Excess concentrated hydrochloric acid is added to a 1.0 M solution of cobalt (II) chloride. Co2+ + 4 Cl- → [Co(Cl)4]220. Liquid bromine is carefully added to a solution of potassium iodide. Br2 + 2 I- → 2 Br- I2