Solution Key for Exam 2
advertisement

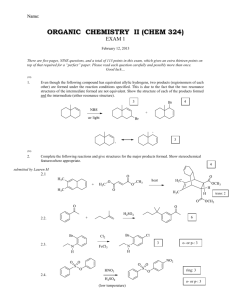
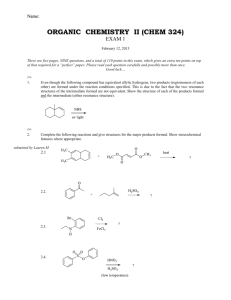
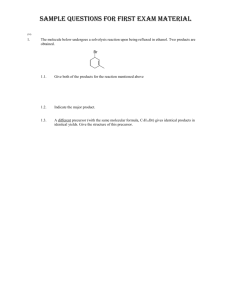
Name: ORGANIC CHEMISTRY II (CHEM 324) EXAM 2 March 12, 2013 There are seven pages, TEN questions, and a total of 114 points in this exam, which gives an extra seventeen points on top of that required for a “perfect” paper. Please read each question carefully and possibly more than once. Good luck… (9) 1. The questions below are about the 1H NMR characteristics of 1,1-dichlorobutane: 1.1. How does the presence of the chlorides alter the electron density around H A? reduces the electron density around HA 1.2. How does the presence of the chlorides alter/influence the energy difference between the two spin states of HA (under a magnetic field)? (your answer to question 1.2 must be consistent with your answer to question 1.1) increases the energy difference 1.3. In the spectrum of 1,1-dichlorobutane below the HB signal is missing. Draw the missing signal as it would be displayed in the spectrum (with the correct splitting pattern). Note the 1:5:10:10:5:1 intensity pattern in the hextet 1.1. In the spectrum of 1,1-dichlorobutane below the HB signal is missing. Draw the missing signal as it would be displayed in the spectrum (with the correct splitting pattern). Chem 324 Exam 2 B. Terem Inspired by questions from Amanda and Merritt (participation by Marcus is gratefully acknowledged) (7) 2. The two “insignia” shown below are designed by future CEO’s (known simply as AManda and MArcus M) of two very prominent pharmaceutical companies. The more conventional structures are given under each insignia. The 1H NMR spectrum of AManda was recorded in the company NMR instrument and displayed at the company head-quarters, as well as below; but the spectrum of MArcus M was not immediately available. Please help your favorite (or second favorite) CEO and explain clearly how the downfield signals (between 4.0 to 5.0 ppm) of MAM are different from the downfield signals of AM. In your explanation refer to the number of signals in the downfield chemical shift range (of the missing spectrum) and to the splitting pattern(s) observed. (Assume that each compound exists as a single stereoisomer) 4.4 4.0 3.6 3.2 2.8 2.4 2.0 1.6 1.2 0.8 0.4 The hydrogens shown are equivalent, and therefore are displayed as one signal. Page 2 They do not split each other since they are equivalent. The only spin-spin coupling is generated by the neighboring methylene hydrogens of the “pentyl” side chain. The signal appears as a triplet Chem 324 Exam 2 B. Terem Inspired by a question from Lauren M (12) 3. Give a “step-by-step” outline for the following multi-step synthesis. Show the products formed for each step. Identify all reagents and any other precursors used. (18) 4. Complete the following reactions. Show all stereoisomers formed if more than one is expected: 4.1. 4.2. 4.3. Page 3 Chem 324 Exam 2 B. Terem Question submitted by Jeanette (5) 5. The compound below has a number of potentially acidic hydrogens, which can be deprotonated under appropriate conditions. Rank the acidity of each hydrogen by placing a number in the boxes provided (“1” for most acidic, “2” next acidic, etc). 5 3 1 2 4 (11) 6. The structure below demonstrates the effect of a substituent in being able to delocalize the charge in a conjugate base. Write an equally stable resonance structure of the conjugate base below (in which no more than three atoms carry a formal charge) 6.1. Write a structure in which the conjugate base is protonated (remember not to alter the stabilizing ability of the substituent) 6.2. The correct structure for 6.1 has a pKa of 7.2. However, a regioisomer of this compound has a pKa of 9.3. Write the structure of the regioisomer (with a pKa of 9.3). 6.3. Explain why the two regioisomers have such different pKa values. The nitro group at the meta-position cannot participate in delocalizing the negative charge of the conjugate base, and thus cannot stabilize the conjugate base (try writing resonance structures) Page 4 Chem 324 Exam 2 B. Terem (14) 7. The structures shown below highlight the spatial arrangements of some of the hydrogen atoms in propylbenzene and styrene. Please answer the questions related to the 1H NMR features of the two compounds based on these spatial features. 7,1, Circle the correct answer about HB and HC in propylbenzene and styrene (1) (2) (3) (4) 7.2. HB and HC are equivalent in both propylbenzene and styrene HB and HC are not equivalent in either propylbenzene or styrene HB and HC are equivalent in propylbenzene only HB and HC are equivalent in styrene only Explain the answer you gave for 7.1. (you might wish also to explain why you ruled out some of the answers you have not chosen) Due to the free rotation in the propyl group, the special relationship of H B and HC to the groups on the benzylic carbon are averaged out, and they end up being in the same chemical environment. On the other hand, because of the absence of free rotation in styrene, HB and HC are in different spatial environments and thus not equivalent. 7.3. Are the chemical shift values of HB and HC the same in propyl benzene? (Circle the correct answer) YES NO 7.4. Are the chemical shift values of HB and HC the same in styrene? (Circle the correct answer) YES NO 7.5. Which of the statements below is the most crucial in explaining that the splitting pattern of HA in propylbenzene is a triplet? Note that J is a symbol for the coupling constant. (1) (2) (3) (4) (5) J(HA-HB) is equal to J(HA-HC) J(HA-HB) is not equal to J(HA-HC) There are three hydrogens in the vicinity of H A The dihedral angle between HA and HB is different than the dihedral angle between HA and HC The splitting pattern of HA in propylbenzene is not a triplet (4) 8. Name the following: 8.1. (common name only) acetic acid 8.2. (common name only) Page 5 formaldehyde Chem 324 Exam 2 B. Terem (18) 9. Circle the best correct answer (only ONE) for each of the multiple-choice questions below. 9.1. Which of the following will display two doublets in the aromatic region of its 1H NMR spectrum? 9.2. Which of the following statements describes the process during the preparation of a Grignard reagent? (1) Lithium is reduced (2) The alkyl halide is reduced (3) Magnesium is reduced (4) Magnesium replaces bromide in a substitution reaction (5) Li or Mg can be used as the oxidizing agent 9.3. Retinal, molecular formula C20H28O, is an aldehyde related to vitamin A, which contains a ring and a number of conjugated double bonds. How many C=C double bonds are there in retinal? (1) three 9.4. (2) four (3) five (4) six (5) seven Identify the reason(s) the acid-base equilibrium given below may favor the products on the right hand side of the equation as written. (A) Benzoate is a stronger base than phenolate (B) Phenol is a stronger acid than benzoic acid (1) only A (2) only B (3) both A and B (4) The equilibrium does not favor the reactants on the right hand side of the equation as written 9.5. Which of the following can be oxidized into a carboxylic acid? (1) only B and C (2) only A and B (3) only C and D (4) only C (5) all of the compounds above, A, B, C, and D, can be oxidized into carboxylic acids 9.6. Which of the following is not the product of an aldehyde or a ketone undergoing a reaction with NaBH4? OH (1) OH (2) Page 6 OH OH (3) (4) Chem 324 Exam 2 B. Terem (16) 10. Each of the products below are formed as a result of a reaction between an organic precursor and a reagent which is specified.. Give the structures of each of the missing organic precursors. 10.1. Inspired by question from Darian 10.2. 10.3. 10.4. Page 7