Solution Key - Chemistry With BT
advertisement

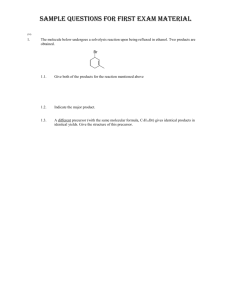
Name: ORGANIC CHEMISTRY II (CHEM 324) EXAM 1 February 12, 2013 There are five pages, NINE questions, and a total of 113 points in this exam, which gives an extra thirteen points on top of that required for a “perfect” paper. Please read each question carefully and possibly more than once. Good luck… (10) 1. Even though the following compound has equivalent allylic hydrogens, two products (regioisomers of each other) are formed under the reaction conditions specified. This is due to the fact that the two resonance structures of the intermediate formed are not equivalent. Show the structure of each of the products formed and the intermediate (either resonance structure). 3 4 3 (24) 2. Complete the following reactions and give structures for the major products formed. Show stereochemical featureswhere appropriate. 4 submitted by Lauren M 2.1 trans: 2 6 2.2. 2.3. 3 o- or p-: 3 ring: 3 2.4. o- or p-: 3 Chem 324 Exam 1 B. Terem (15) 3. Circle the best correct answer (only ONE) for each of the multiple-choice questions below. 3.1. Which of the following can be converted into benzoic acid upon reaction with KMnO 4? (1) They can all be converted into benzoic acid upon reaction with KMnO 4 (2) A, B, and D (3) only B (4) B and C (5) B, C, and D 3.2. Which of the following is most likely to undergo bromination in the absence of a Lewis acid?: 3.3. In which cyclic molecule(s) below are all C-C bond lengths the same? (1) both A and B 3.4. 3.5. (2) both A and C (3) only A (4) only B (5) only C Which of the following is called benzyl alcohol? OH NH2 CH2OH OCH3 (1) (2) (3) (4) Which of the following does not give a reaction product upon treatment with HCl? (1) only A (2) A and B (3) B and C (4) A and C (5) none of the compounds above gives a reaction product upon treatment with HCl Page 2 Chem 324 Exam 1 B. Terem (11) 4. Identify the structures of the missing starting materials and/or products: 4.1. 3 2 When a diene is treated with HCl at a reaction temperature below 0 oC, the product shown below is formed. However, at higher reaction temperatures a different major product is formed. Identify the structures of the missing diene and the missing product. 4.2. 3 3 (9) 5. Phenyl acetate and methyl benzoate are constitutional isomers of each other, and they are both esters. However, one of them undergoes nitration much faster than the other one and at a lower reaction temperature. 5.1. Assuming that the reaction profile below illustrates the different stages (designated by letters a to e) of the reaction during nitration for either of the esters above, circle the letter, whose energy level determines the rate of the reaction. 3 6 benzoate: 2 (only if m-) 5.2. Decide which of the esters above undergoes nitration faster and draw its structure as it resembles the species you indicated (circled) in the previous question (5.1) (Note that you will have to get question 5.1 right in order to answer 5.2) Page 3 Chem 324 Exam 1 B. Terem (8) 6. In the compound below “W” is a hypothetical substituent which has a lone pair and is capable of stabilizing the -intermediate by resonance (through -bonds) in electrophilic aromatic substitution reactions. Draw two resonance structures for the -intermediate formed in the acylation reaction below, one of which should clearly show the stabilizing effect of the substituent “W”. 4 4 (15) 7. Show a correct sequence of reactions for the multiple-step syntheses below. Indicate all reagents, reaction conditions, and show the structures of the intermediate products. 7.1. 1 1 1 2 3 7.2. 1 1 2 3 Page 4 Chem 324 Exam 1 B. Terem (10) 8. Complete the reaction below: Show details of mechanism for each step of the reaction (starting from the initiation step). Use curly arrows. 1 2 uv 2 O O H Cl O + Cl OH 2 H H Cl Cl Cl Cl 1 2 2 (8) 9. Give a brief explanation for each of the questions below: 9.1. Cantharidin, whose structure and stereochemical features are shown below, is a natural product which can be isolated from certain species of beetles. It is highly toxic and (falsely) rumored to possess “aphrodisiac” activity. The immediate precursor (on the left side of the equation) looks like a Diels-Alder product; however, it is not possible to obtain this product through a Diels-Alder reaction. Explain (in a few words). 9.2. Methyl benzoate can be converted into methyl m-nitrobenzoate at a reaction temperature lower than 15oC. Why is a dinitration product formed only at higher temperatures? Page 5 Chem 324 Exam 1 B. Terem 1 2 3 4 5 6 7 8 9 10 11 12 13 10 24 15 11 9 8 15 10 8 0 0 0 0 TOT 110 Page 6