Heat Lab
advertisement

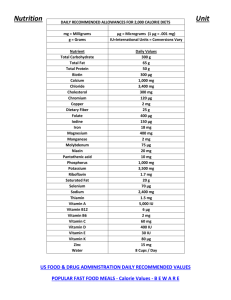
Name: Per: Metabolism Lab I. Introduction A. Food Energy Energy is divided in two major classes: potential (PE) and kinetic energy (KE). Potential energy is energy stored in matter, kinetic energy is energy stored in motion. The units for both are 1 cal (older) or Joule (newer). Write down the conversion factor to change units into each other. Food energy is the same as chemical energy, both belonging to the chemical energy class. Due to the large amount of calories in food, the food calorie is actually a kilocalorie and has a capitalized unit 1 Cal = 1000 cal which is 1 Cal = 4.18 kJ The maximum amount of energy found with a particular food can be measured by burning the food in a calorimeter. In a calorimeter, the energy from combusting a food item is transferred to water which has a very defined specific heat capacity of 4.18 J/goC. Absolute food energy is usually higher than the energy a body can extract from food since food is a combination of caloric and non-caloric nutrients. Non-caloric nutrients are water, fibers, vitamins, minerals, antioxidants, caffeine, spices and natural flavors. Nutritionists talk about the number of calories in a gram of a nutrient. Fats and alcohol have the greatest amount of food energy: 8 Cal/g, proteins and carbohydrates are lower: 4 Cal/g. An example of a high energy but no-caloric nutrient for humans is the structural polysaccharide cellulose found in fruits and vegetables. The human digestive system lacks the enzyme cellulase and can therefore not utilize the energy stored in this molecule which is therefore termed dietary fiber. B. Food Labels Additionally, each food has a specific Metabolic Energy Intake (MEI), which is only 85% of its theoretical caloric value. MEI is the amount of energy a cell can extract via cellular respiration minus any energy lost from digestion (chewing, swallowing, digestive juice production, active transport etc). Approximately 15% of the total energy is lost this way and is converted to heat. Below is the nutritional label of Basmati rice. The FDA requires food manufacturers to label the energy content of their products, to help consumers monitor their caloric intake. The caloric content of food is usually given on labels for 100 g and/or for what the manufacturer claims is a typical serving size. Table A. Energy Density Food Type kJ/g kcal/g Fat 37 9 Ethanol (alcohol) 29 7 Proteins 17 4 Carbohydrates 17 4 Organic acids 13 3 Polyols (sugar alcohols, sweeteners) 10 Fibers 8 2.4 2 Name: Per: C. Calories per Day Recommended caloric intake for men is 2500 Cal/day and 2000 Cal/day for women. Children, sedentary and older people require less energy, physically active people more. Calories taken up but not used are stored either in the liver in form of glycogen or as fat in the adipose tissue. D. Efficiency of Machines The conversion of chemical energy into the physical work of muscles is further reduced by the limitations of the mechanical machinery. The efficiency of converting respiration energy into mechanical energy is only about 40%, a number slightly higher than that of a car engine which is about 38%. The difference in energy is lost in form of heat, rising the body temperature of humans to 37oC. E. Work and Energy Altitude/height belongs to the gravitational energy class. It requires work to lift an object or to climb stairs. The amount of work (W) depends on the mass (m) of the object, the height (h) it is lifted to and gravitation (g) of the location resulting in the formula W = m x g x h Gravity as the unit m/sec2, mass the unit kg, and height the unit m. Substitute the units for each variable and find the composite unit of PE, which equals the substitute unit Joule W = mass x gravity x height = ____ x _____ x _____ = _________ = 1 Joule II. PreLab 1. The food label from Flamin' Hot Cheetos is shown to the right. Have you ever eaten these, and if so...how often? 2. List the % of total fat, total carbs and proteins 3. If you consume 1 serving of this food how many grams of fat, carbs and proteins would you consume? 4. Use the conversion key from table A. and convert the grams from fat, carbs and proteins into calories and add them up. Show math!!! Compare your calculated calories with the calories per serving on the label? Do they compare? 5. Does this food contain any vitamins, if yes, list them 6. The total amount of fat is not as important as what types of fat the product contains. List the % of fat types present. Name: Per: 7. A balanced nutritional rule of thumb is considered the 80/20 split, meaning that 80% of your food should be healthy, and 20 % should be fun. In which catogory does your food item fall under? III. Procedure/Results A. Calorimetry of Food 1. Obtain a balance and calibrate it to zero. 2. Mass the two food itema and enter in the table under initial mass = mi. 3. Using your graduated cylinder measure 20 ml of water 4. Pour the water into a 50 ml Erlenmeyer Flask. 5. Attach the flask to the clamp on the ring stand. 6. Using a thermometer measure the temperature of the water and record under initial temperature Ti 7. Attach the Cheerio on the food holder. Center the Erlenmeyer directly over the food item and light it on fire, carefully! 8. When the food item stops burning, stir the water in the Erlenmeyer for about a minute with the thermometer and then take the final temperature Tf, record. 9. Carefully remove the remains of the food item onto a weigh paper, tare with an empty weigh paper and mass again. Record as final mass mf 10. Calculate the change in mass ∆ m 11. Repeat with the half peanut. Data Table I: Mass of Food Items Food item Initial mass = mi Cheerio ___________g ½ Peanut ___________g Final mass = mf Change in mass: ∆m= mi - mf 0.02 g __________g 0.20 g __________g Data Table II: Temperature of Water Food item Initial Temp = Final Temp = Ti Tf Change in Temp: ∆T = Tf - Ti Cheerio ½ Peanut Data Table III: Calculate the Heat Q That Was Released by the Burning Food Q = m x C x ∆T C water = 1 cal/g0C Food item Cheerio Mass of 20 ml water mwater Change in Temp: ∆T (table II) Heat Q ½ Peanut 20 g 12. Find the Heat Released Per Gram of the Food Item How much heat energy was stored in each gram of each of the food items? cal Name: Per: Calculation: Q (per gram) =_Q (table III) ∆m (table I) Table IV: Heat energy stored in food item per gram Food item Q ( table III) Change in mass of food: mi - mf (tableI) Cheerio cal g Peanut cal _________g Energy stored per gram food item cal g cal g B. Workout Burn 1. Take a ruler and go to the bottom of a flight of stairs 2. Measure the height of one stair in cm, record, convert to meters (1m = 100 cm) 3. Now walk up the flight of stairs counting how many stairs you are climbing to the top, record 4. Calculate the total height gain by multiplying the number of stairs with the height of one stairs, record 5. Record your body weight in kg. 1 lbs = 0.4 kg 6. Calculate the potential energy PE you gained by climbing the stairs using the formula from Introduction D. Gravity on earth is 9.8 m/sec2. Show math. Height of 1 stair _____ cm = ______ m Number of stairs _____ Total height of entire flight of stairs ______ x _______ = _______ Body weight _______ kg PE = m x g x h = ______ x _______ x ______ = ____ ______= ______J IV. Discussion Questions a. For both food items copy how many carbs, proteins and lipids and calories each serving has Cheerio Peanuts b. Describe the difference in burning time and intensity for both food items ___________________________________________________________________________________ ___________________________________________________________________________________ c. How does the content of both food items explain the different burning times? ___________________________________________________________________________________ ___________________________________________________________________________________ Which food item released the highest amount of heat energy Q? ________ d. Explain how the calories stored in a food item related to the burning time of the item? ___________________________________________________________________________________ ___________________________________________________________________________________ e. Calculate how many peanuts and Cheerios do you need to eat to make up for the amount of energy expended by climbing the flight of stairs. Clearly show work for each case: