Self Evaluation 1 Key - Chemistry at Winthrop University
advertisement

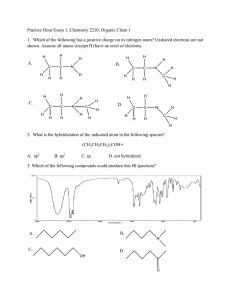
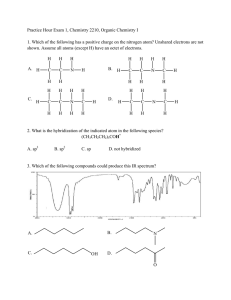
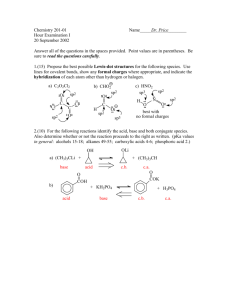
CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 1 1. Atoms and Orbitals a. Using horizontal lines and arrows, draw an accurate ground state electronic configuration of silicon. Label each orbital and clearly show relative energies. 3p 3s 2p 2s 1s b. Provide the orbital hybridization of the indicated atoms. sp2 CH3 sp H H C C C H C CH3 H H CH2 C H CH3 sp C C sp3 sp2 H O CH2CH3 C H sp3 sp3 H3C CH3 CH3CH2 sp3 3 sp c. Describe the two orbitals that overlap to form the bonds indicated by each arrow. H B H C+D E (s - sp3) A _________________ (s -sp2) B _________________ (p - p) C _________________ (sp2 - sp2) D _________________ * C + D comprise a double bond. Assign C as the weaker bond of the two. (sp3 - sp3) E _________________ 1 CH2CH3 CH2CH3 sp2 A N CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 1 2. Lewis Structures Draw complete Lewis structures for each of the following species. C3H5F LiAlH4 CH3CH2NO2 H Li+ H Al H H H H H C C H H N2O (No Charges) H O N F C N H O C H N O or C H N H N O 3. Nomenclature Give the proper IUPAC name for each of the following structures on the lines provided. O CH3CH2CH2 1-ethyl-2-(2-methylpentyl)cyclohexane CH2CH(CH3)2 2-methyl-1-propoxypropane Br 3-bromo-5methyl-4-(1-methylethyl)heptane Br OCH2CH3 Br OH 5-methyl-3-hexanol 8-ethoxy-4-ethyl-1,6-nonadiene 3,3-dibromocyclooctene OH CH3 CH2CH2CHFCH2CH3 N C C 3-ethyl-5-methyl-1-heptyne H 2-(1-methylethyl)-1-hepten-4-ol 2 H 3-fluoro-N-methyl-1-pentanamine CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 1 4. Terminology For each compound below, indicate the degree of substitution at the carbon indicated with an arrow. Identify the class of each compound (amine, alkyl halide, etc.) and the type as appropriate for the class (primary, internal, disubstituted, etc.). 2o methyl 4o OH 3o CH3 N Cl H alkyl halide amine alkene (cycloalkene) Class Class tertiary secondary disubstituted primary Type Type Type Type alcohol Class Class 5. Dipoles and Physical Properties a. Use symbols (δ+ and δ-) to designate the dipoles of the indicated bonds for each molecule. Rank the bonds in each set in order of decreasing polarity (1 most polar to 3 least polar). Na H Li 2 H K H 1 3 F none Cl Cl NH2 1 3 O 4 2 CH3 N H 1 5 3 F OCH3 2 b. Rank each from HIGHEST boiling point (1) to LOWEST boiling point (5). 3 CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 1 6 . Application of Concepts a. Before you are three bottles, each containing a clear, colorless liquid. One bottle contains pentane, another contains heptane and the remaining contains 1-hexanol. Using concepts discussed so far in class, how can you determine which compound is in each bottle? Explain your plan and rationale. OH Because of the differences in size and functional groups, these compounds will have very different boiling points. One could simply determine the boiling point of the liquid from each bottle, and know that the highest boiling must be 1-hexanol (large and capable of hydrogen bonding) and the lowest boiling must be pentane (smallest and lacking other strong intermolecular forces). The remaining liquid is therefore heptane. b. Draw the structure of the only acyclic (no rings) 7-carbon tertiary alcohol that also has a quaternary carbon. OH c. Draw the Lewis structure for methanamine hydrochloride (CH3NH3+ Cl-). H H H C N H H H Cl How many electrons are in this compound? 36 electrons How many valence electrons are in the cation? 14 electrons Assuming the most abundant isotopes, how many neutrons are found in this compound? _31_ How many molecular orbitals are in the cation? 14 MOs (7 bonding and 7 antibonding) How many total bonding interactions are there in this compound? 8 (7 covalent and 1 ionic) 4