Exam 1 yellow white
advertisement

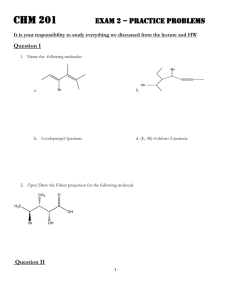
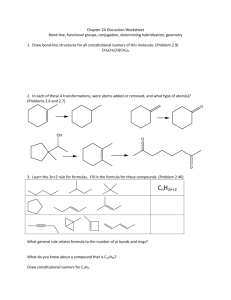
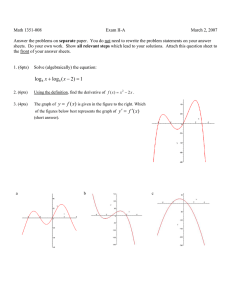
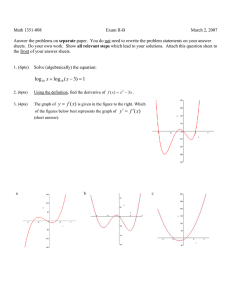
Name_____________________________________________________ Exam 1 R340 Fall 2014 This exam contains 104 points and will be scored out of a maximum of 100 points. 1. (8pts) Draw a bond-line formula for each of these molecules, then indicate whether you would consider each of these atoms to be Lewis acidic or Lewis basic. a. The carbonyl carbon atom of a cyclic ketone b. The oxygen atom of a saturated alcohol 2. (14pts) On the structure below, A. Label three functional groups on the structure below. B. Indicate the hybridization of the heteroatoms on the structure below. C. How many lone pairs in this molecule? __________ D. How many hydrogen atoms in this molecule? __________ 3. (6pts) Circle the aromatic compound(s), and put an X through any that are not aromatic. 4. (14pts) Although there are 4 areas of electron density around the nitrogen atom in the Lewis dot structure below, the nitrogen atom bond angles are 120o. A. Draw a simplified orbital overlap picture for this molecule. B. How does your picture help explain why the bond angles are 120o? C. How many lone pairs are orthogonal to the pi system?__________ D. How many electrons are in the pi system? _____________ E. Is this molecule aromatic? _______________ 5. (12pts) For each of these structures, draw all significant resonance structures. If there are no significant resonance structures, write “no significant structures.” Blank answers are incorrect. 6. (6pts) Which of these aromatic systems is “electron rich” compared to benzene? Circle it, and draw one resonance structure that shows an electron rich benzene ring. 7. (12pts) Indicate which of the following lone pairs is more stable, and circle the physical reason it is more stable. 8. (8pts) Draw bond-line structures of both enantiomers of 2,3,6-trimethylhept-4-yn-2-ol . Use appropriate bond angles in your bond-line formula. 9. (8pts) Draw dipole arrows designating any polar bonds in these molecules. Indicate any carbon atoms that are Lewis acidic. 10. (8 pts) Fill in the boxes: 11. (8pts) For the following molecule, fill in the boxes as E/Z/R/S or put an “X” in the box if none of the labels are appropriate. PERIODIC TABLE OF THE ELEMENTS 1 H 1.008 3 Li 6.941 11 Na 22.99 19 K 39.10 37 Rb 85.47 55 Cs 132.9 87 Fr (223) 4 Be 9.012 12 Mg 24.31 20 Ca 40.08 38 Sr 87.62 56 Ba 137.3 88 Ra (226) Scratch work: 1 Atomic Number H Symbol 1.008 Atomic Weight 5 B 10.81 13 Al 26.98 21 22 23 24 25 26 27 28 29 30 31 Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 39 40 41 42 43 44 45 46 47 48 49 Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 57 72 73 74 75 76 77 78 79 80 81 La Hf Ta W Re Os Ir Pt Au Hg Tl 138.9 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.6 204.4 89 Ac (227) 6 C 12.01 14 Si 28.09 32 Ge 72.61 50 Sn 118.7 82 Pb 207.2 7 N 14.01 15 P 30.97 33 As 74.92 51 Sb 121.8 83 Bi 209.0 8 O 16.00 16 S 32.07 34 Se 78.96 52 Te 127.6 84 Po (209) 58 Ce 140.1 90 Th 232.0 69 Tm 168.9 101 Md 70 Yb 173.0 102 No 71 Lu 175.0 103 Lr 59 Pr 140.9 91 Pa (231) 60 Nd 144.2 92 U 238.0 61 Pm (145) 93 Np 62 Sm 150.4 94 Pu 63 Eu 152.0 95 Am 64 Gd 157.3 96 Cm 65 Tb 158.9 97 Bk 66 Dy 162.5 98 Cf 67 Ho 164.9 99 Es 68 Er 167.3 100 Fm 9 F 19.00 17 Cl 35.45 35 Br 79.90 53 I 126.9 85 At (210) 2 He 4.003 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.3 86 Rn (222)