Stereochemistry
advertisement
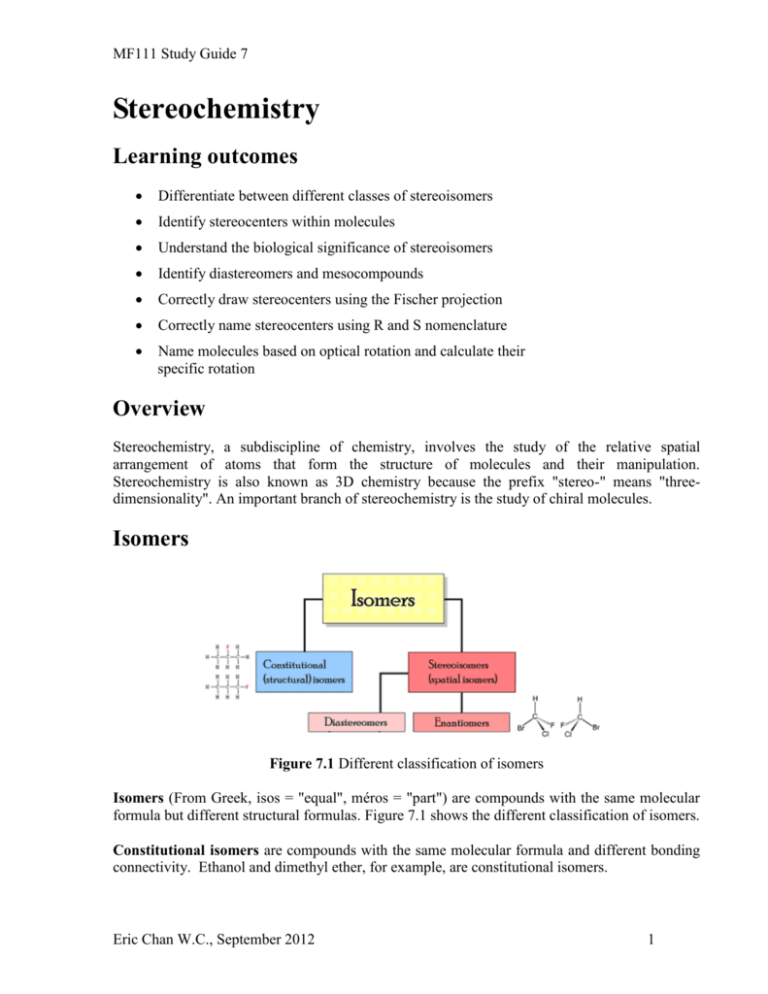
MF111 Study Guide 7 Stereochemistry Learning outcomes Differentiate between different classes of stereoisomers Identify stereocenters within molecules Understand the biological significance of stereoisomers Identify diastereomers and mesocompounds Correctly draw stereocenters using the Fischer projection Correctly name stereocenters using R and S nomenclature Name molecules based on optical rotation and calculate their specific rotation Overview Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. Stereochemistry is also known as 3D chemistry because the prefix "stereo-" means "threedimensionality". An important branch of stereochemistry is the study of chiral molecules. Isomers Figure 7.1 Different classification of isomers Isomers (From Greek, isos = "equal", méros = "part") are compounds with the same molecular formula but different structural formulas. Figure 7.1 shows the different classification of isomers. Constitutional isomers are compounds with the same molecular formula and different bonding connectivity. Ethanol and dimethyl ether, for example, are constitutional isomers. Eric Chan W.C., September 2012 1 MF111 Study Guide 7 Figure 7.2 Two constitutional isomers Stereoisomers are compounds that have the same molecular formula and the same atom-to-atom connectivity, but have a different arrangement of atoms in space and cannot be interconverted by rotation about a single bond Figure 7.3 Two stereoisomers of alanine Figure 7.3 shows the two stereoisomers of alanine. The stereoisomer shown on the left-hand side of this figure, called L-alanine, which is found in virtually all proteins, in all forms of life on earth. The D-alanine isomer is quite rare. Stereochemistry is a branch of chemistry that deals with these isomers. Chirality Louis Pasteur could rightly be described as the first stereochemist, having observed in 1849 that salts of tartaric acid collected from wine production vessels could rotate plane polarized light, but that salts from other sources did not. In 1874, Jacobus Henricus van 't Hoff and Joseph Le Bel explained optical activity in terms of the tetrahedral arrangement of the atoms bound to carbon. Chiral carbons have with four different substituent groups are the most common stereocenters in organic molecules (Figure 7.4). Figure 7.4 Two mirror images of a chiral carbon. The term chiral is derived from the Greek word for “handedness” – ie. right-handedness or lefthandedness. Your hands are chiral: your right hand is a mirror image of your left hand, but if you place one hand on top of the other, both palms down, you see that they are not superimposable (Figure 7.5). Eric Chan W.C., September 2012 2 MF111 Study Guide 7 Figure 7.5 Chirality of hands Enantiomers Enantiomers (From Greek, enantios = “opposite”, méros = "part") are stereoisomers that are mirror images of each other and are non-superposable (not identical). Figure 7.6 shows some examples of chiral molecules that exist as pairs of enantiomers. In each of these examples, there is a single stereocenter, indicated with an arrow. Many molecules have more than one stereocenters and these are classified differently. A racemic mixture is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. Figure 7.6 Common enantiomers Eric Chan W.C., September 2012 3 MF111 Study Guide 7 Biological significance Drug safety Thalidomide is a drug, first prepared in 1957 in Germany, prescribed for treating morning sickness in pregnant women. Thalidomide had previously been used in other countries as an antidepressant, and was believed to be safe and effective for both purposes. The effective isomer is (R)-thalidomide while (S)-thalidomide was found to be a teratogen i.e. causes malformations of an embryo or fetus (Figure 7.7). (S)-thalidomide (R)-thalidomide Figure 7.7 (R)-thalidomide and (S)-thalidomide. Both have chiral carbons and cannot be super imposed. However, it is incorrect to say that (R)-thalidomide is safe for pregnant women but (S)-thalidomide is not. It was discovered (too late) that the the drug undergoes racemisation in the human body i.e. even if only one of the two enantiomers is administered as a drug, the other enantiomer is produced as a result of metabolism. The thalidomide scandal led to enactment of much stricter laws requiring tests for safety during pregnancy. Drug patents Pharmaceutical companies regularly patent potential drugs to ensure exclusive rights to the drug for a limited period of time in exchange for public disclosure of the drug formulation. Advances in industrial chemical processes have made it possible for pharmaceutical companies to extend the patents of drugs originally marketed as a racemic mixture by patenting individual enantiomers (Figure 7.8). In some cases, the enantiomers have genuinely different effects. In other cases, there may be no clinical benefit to the patient, except to extends the drug's patentability. Eric Chan W.C., September 2012 4 MF111 Study Guide 7 Figure 7.8 Patenting of racemic Ibuprofen and S-Ibuprofen Diastereomers Diastereomers (sometimes called diastereoisomers) are stereoisomers that are not enantiomers (Figure 7.9). Diastereomerism occurs when there are two or more possible stereoisomers of a compound. Each stereocenter gives rise to two different configurations and thus increases the number of stereoisomers by a factor of two (2n). We will mainly concentrate on smaller compounds with two to three stereocenters. Eric Chan W.C., September 2012 5 MF111 Study Guide 7 Enantiomers Diastereomers Diastereomers Enantiomers Figure 7.9 Enantiomers and diastereomers of erythrose Mesocompounds are rare exceptions which have or more stereocenters but the compound itself is chiral i.e. no enantiomers. A meso compound has enantiomers because it is superimposable on its mirror image despite having chiral carbons (Figure 7.10). Mesocompounds are optically inactive and there are very few naturally occurring examples. Meso-tartaric acid is one such example. one not no Figure 7.10 Mesocompound of tartaric acid and its diastereomers Figure 7.11 Cyclic mesocompound Cyclic compounds can also be meso. One of many such examples is cis-1,2dihydroxycyclohexane (Figure 7.11). However, if the hydroxyl groups are trans to each other, the molecule is chiral. Eric Chan W.C., September 2012 6 MF111 Study Guide 7 Cis-trans isomers of alkenes occur because the double bonds in molecules do not allow rotation. Therefore, the isomers cannot interconvert without breaking any bonds. These isomers are sometimes classified as diastereomers i.e. stereoisomers that are not enantiomers. When there are only two substituent groups, the nomenclature for these isomers are straight forward i.e. cis when substituent groups are on the same side, trans when the stubstituent groups are on different sides. Cis and trans isomers can have a very different shape Figure 7.12. You may want to read up the E/Z nomenclature for alkenes with more than two substituent groups. Figure 7.12 Cis and trans fatty acids. Trans fatty acids have a much straighter carbon chain and tend to have higher boiling points. Stereoisomer nomenclature Drawing stereocenters Fischer projection is a simplified way to depict the stereochemistry around a stereocenter. The simpler hashed-wedged line perspective drawings are effective, but can be troublesome when applied to compounds having multiple chiral centers. As part of his Nobel Prize-winning research on carbohydrates, the great German chemist Emil Fischer, devised a simple notation that is still widely used. In a Fischer projection drawing, the four bonds to a chiral carbon make a cross with the carbon atom at the intersection of the horizontal and vertical lines. The two horizontal bonds are directed toward the viewer (forward of the stereogenic carbon). Naming stereocenters Eric Chan W.C., September 2012 7 MF111 Study Guide 7 Chemists need to have a convenient way to distinguish between stereoisomers. The Cahn-IngoldPrelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations ‘R’ (from the Latin “rectus”, meaning right-handed) or ‘S’ (from the Latin “sinister”, meaning left-handed). Trivia: Sinister also means “unlucky” in latin and this led to the negative associations with the word “left”. Using the system, substituents bound to a chiral carbon can be assigned priorities based on their atomic number. Substituent groups with a higher atomic number are assigned a higher priority. Figure 7.13 shows how the priority of each substituent group of the chiral carbon in glyceraldehyde is assigned. Priorities of the substituent groups are: 1. O (atomic number 8; hydroxyl) 2. C (atomic number 6; aldehyde) 3. C (atomic number 6; CH2OH) 4. H (atomic number 1), Figure 7.13 Priorities for substituent groups in chiral carbon of glyceraldehyde The priority of the H and O groups are easily assigned based on their atomic numbers. The priorities of the C groups require more deliberation. When this occurs, we have to look at atoms bonded to the C groups and determine which has the largest atomic numbers. However, in the case of glyceraldehyde, both C groups are bonded to O. In such cases, a double bond is assigned a higher priority than a single bond. Figure 7.14 Determining the “handedness” of the chiral carbon. To determine the handedness of the molecule, we have to flip the molecule so that the group with the lowest priority (usually H) is facing away from us. Then we have to trace a circle Eric Chan W.C., September 2012 8 MF111 Study Guide 7 around the groups assigned priority 1, 2 and 3 and determine if the circle is turning clockwise or counter clockwise. Figure 7.14 shows that the glyceraldehydes molecule in Figure 7.13 is of the R configuration i.e. (R)-glyceraldehyde. For (S)-glyceraldehyde, the circle around the 1, 2, and 3 priority groups is counter-clockwise (Figure 7.15). Again, remember to flip the molecule so that the group with priority 4 is pointing to the back. Figure 7.15 “Handedness” of (S)-glyceraldehyde Optical Activity Electromagnetic waves, such as light waves exhibit polarization because they can oscillate with more than one orientation (Figure 7.16). Figure 7.16 Oscillation of unpolarized light Light passing through a polarizer produces plane polarize light i.e. oscillates on a single plane. Optically active substances are able to rotate the plane of polarised light and this rotation is detected and measured in a polarimeter (Figure 7.17). Eric Chan W.C., September 2012 9 MF111 Study Guide 7 Figure 7.17 Schematic diagram of polarimeter After polarised light is generated it passes through the sample cell (Figure 7.18). After emerging from the cell, the light passes through a second polarising filter (analyser) and finally the detector (often your eyes). If the sample is optically inactive, the plane is unchanged and no rotation is detected. If the sample is optically active, the plane of polarisation is rotated. The polarimeter is used to determine both the direction and magnitude of the rotation Figure 7.18 Polarimeter and sample cell Eric Chan W.C., September 2012 10 MF111 Study Guide 7 Compounds are classified based on how the rotate polarized light: Dextrorotatory (d) or (+): Clockwise rotation is assigned a positive value Levorotatory (L) or (-): Anticlockwise rotation is assigned a negative However, the magnitude of the rotation varies depending on the length of the sample cell and concentration. Therefore the specific rotation as calculated below is required for comparison between different experiments: [ ] c α is observed rotation [α] is specific rotation ℓ is path length in dm c is concentration in g/mL It is important to note that (+) and (-), and (d) and (l) assignments tell us nothing about the structure of the molecule. It is important to not confuse them with (R) and (S) assignments which can be assigned simply by looking at the structure. The way to assign (+) or (-) to a molecule is to place it in a polarimeter! However, assignments based on optical rotation e.g. (d)-glucose is convenient for substances with multiple stereocenters (Figure 7.19). Figure 7.19 (d)-glucose can also be classified as (2R, 3S, 4R, 5S)-glucose Question: How many stereoisomers does glucose have? Reading Material Chapters 15.2 Silberberg, M.S. (2006). Chemistry: The Molecular Nature of Matter and Change. 4th Ed. McGraw Hill. Eric Chan W.C., September 2012 11





