CHEMISTRY I
advertisement

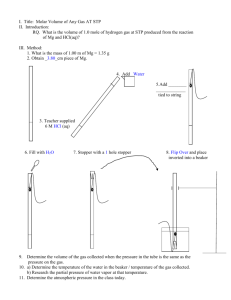
CHEMISTRY I Chapter 11 Gas Laws Name____________________ Worksheet 1. Pressure Unit Conversions. 1. 780 mm Hg is how many kilopascals? 2. How many torr is 600 mm Hg? 3. Convert 486 kPa to mm Hg. 4. 486 kPa is how many atmospheres? 5. How many atm would equal 750 mm Hg? Worksheet 2. Temperature Conversions. 1. Convert the following temperatures to Kelvin. a) 0.0oC b) 27.0oC c) -50.0oC d) -273.0oC 2. Convert the following temperatures to Celsius. a) 273 K b) 350.0 K c) 100.0 K d) 20.0 K e) 0.0 K Worksheet 3. Pressure of gases collected over water 1. What is the pressure of a gas in a eudiometer with a water column 15 mm higher than the water level in the pan if the barometric pressure is 735 mm Hg and the temperature is 25 degrees C? 2. Atmospheric pressure is 720.0 mm Hg. The water column in a eudiometer is 18 mm below the pan level. Temperature is 22 degrees C. What is the pressure of the dry gas? 3. Atmospheric pressure is 780 mm Hg; temperature is 20.0 degrees C. Water column is 27.0 mm above pan level. What is the pressure of the dry gas? 4. Atmospheric pressure is 620 mm Hg; temperature is 25 degrees C. Water column is 35 mm below pan level. What is the pressure of the dry gas? Worksheet 4. Combined Law with Gases Collected over Water 1. A sample of gas is collected over water on a day with the barometric pressure is 790.0 mm Hg and the temperature is 20.0 degrees C. The volume collected is 40.0 mL. What volume would this (dry) gas have at standard conditions? 2. A sample of gas occupying 5.000 mL is collected over water when barometric pressure is 1.35 atm and temperature is 10.5 degrees C. What volume will the gas occupy at STP? Worksheet 5. 1. 2. 3. 4. 5. Density and Molar Volume If the density of a gas is 1.43 g/L at STP, what is the molecular mass of the gas? If the density of a gas is 1.25 g/L at STP, what is the molecular mass of the gas? The mass of 1.00 L of a gas is 4.28 g at STP. What is the molecular mass? What mass would 500.0 mL of nitrogen gas have at STP? What mass would 325 mL of oxygen gas have at STP? Worksheet 6. Ideal Gas Law 1. If 0.531 g of a gas occupies 1.00 l at STP, what is the molecular mass of the gas? 2. Find the mass in grams of 2.50 L of N2,H2,and O2 at STP. 3. How many liters will 5.0 g of oxygen gas occupy at STP? 4. What volume will 50.0 g of fluorine gas occupy at STP? 5. What volume will 5.0 g of oxygen gas occupy at 20.0 C & 745 mm Hg? 6. What volume will 50.0 g of fluorine gas occupy at 25.0C & 780 mmHg? 7. What volume will 2.8 g of hydrogen gas occupy at15.0C & 600.0 mmHg? 8.What volume will 45 g of argon occupy at 25 C and 742 mm Hg? 9.If 300.0 mL of a gas has a mass of 0.528 g at STP, what is the molecular mass of the gas? 10.If 800.0 mL of a gas has a mass of 0.213 g at STP, what is the molecular mass of the gas? 11. If 0.531 g of a gas occupies 1.00 l at STP, what is the molecular mass of the gas? Worksheet 7. Empirical and Molecular Formulas Analysis of a gas reveals it to contain 46.7% nitrogen and 53.3% oxygen. Its density is determined to be 1.35 g/L. What is its empirical formula and what is its molecular formula? Worksheet 8. Cumulative Review 1. A sample of oxygen gas in a 2000.0 mL container has a pressure of 405 kPa. Calculate the pressure, in kilopascals, this gas would have if it were transferred to a container having a volume of 1500.0 mL at the same temperature. 2. Hydrogen is collected in a eudiometer tube, over water, at a temperature of 20.0 degrees C. A manometer indicates that the gas is exerting a pressure of 765.0 mm Hg. What is the pressure of the dry gas? 3. A sample of chlorine gas is contained in a 700.0 mL bottle at a temperature of 50.0 degrees C and a pressure of 101.3 kPa. Calculate what temperature, in degrees Celcius, this gas would have if the volume was reduced to 400.0 mL and the pressure kept constant. 4. A gas occupies a volume of 6240 mL at a temperature of 25.0 degrees C. Calculate the volume it would occupy at 40.0 degrees C if the pressure does not change. 5. A 2700.0 mL container of argon is exerting a pressure of 355 kPa while at a temperature of 273 degrees C. What pressure would this same amount of gas have in a 1350.0 mL container at a temperature of 0.0 degrees C? 6. A sample of gas occupies 300.0 mL at STP. Calculate the volume this same amount of gas would occupy at a pressure of 93 kPa and a temperature of 20.0 degrees C? 7. A gas collected on a day when the atmospheric pressure of 1.12 atm had a volume of 252.4 mL. A day later, that volume had changed to 248.8 mL. What was the atmospheric pressure on the second day? 8. The volume of a dry gas originally at STP was recorded as 488.8 mL. What volume would the same gas occupy when subjected to a pressure of 100.0 atm and a temperature of -245 degrees C? 9. A sample of gas occupying 25.00 mL is collected over water when the atmospheric pressure is 762.00 mm Hg and the temperature is 25.0 degrees C. What is the pressure of the dry gas? What volume will the gas occupy if the pressure is changed to 730.0 mm Hg and the temperature is changed to 30.0 degrees C? 10. What volume would be occupied by 25.0 mL of gas collected over water when the temperature is 28.0 degrees C and atmospheric pressure is 758.0 mm Hg if the temperature is increased to 40.0 degrees C? 11. Find the mass in grams of 2.50 L of N2,H2,and O2 at STP. 12.How many liters will 5.0 g of oxygen gas occupy at STP? 13.What volume will 50.0 g of fluorine gas occupy at STP? 14.What volume will 5.0 g of oxygen gas occupy at 20.0 C and 745 mm Hg? 15.What volume will 50.0 g of fluorine gas occupy at 25.0 C and 780 mm Hg? 16.What volume will 2.8 g of hydrogen gas occupy at 15.0 C and 600.0 mm Hg? 17.What volume will 45 g of argon occupy at 25 C and 742 mm Hg? 18.A compound contains 27.3% carbon and 72.7% oxygen. Its density is 1.96 g/L. Find the empirical formula. Find the molecular formula. 19.If 2.00 L of a certain gas, collected at 780.0 mm Hg and 30.0 C, has a mass of 1.50 g, find the molecular mass of the gas. 20.A gas having a mass of 1.20 g has a volume of 252 mL at 75.0 C and 750.0 mm Hg. What is the molecular formula? 21.Hydrogen gas and oxygen gas combine to form water vapor. If 2.0 L of hydrogen gas and 2.0 L of oxygen gas are used, what is the limiting reagent? How much water is produced? 22.Carbon monoxide burns in air to produce carbon dioxide. If 2.53 L of carbon monoxide are burned at STP, how many grams of carbon dioxide are produced? 23.What volume would the mass of carbon dioxide produced in the question above have at 746 mm Hg and 2 degrees C? 24. What volume would 25.0 g of carbon dioxide occupy at 742 mm Hg and 25 degrees C?