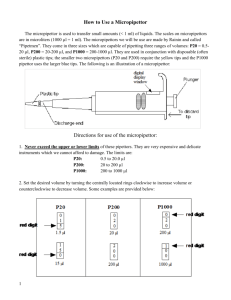
ปรียชาติ จันทโชติและปราโมทย์ ธรรมรัตน์
advertisement

เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) ปรี ยชาติ จันทโชติและปราโมทย์ ธรรมรัตน์ หน่วยสร้ างสานึกและพัฒนาประโยชน์จาก เอกสารสิทธิบตั รเพื่อการวิจยั และพัฒนา (สสวพ) สกว สถาบันค้ นคว้ าและพัฒนาผลิตภัณฑ์อาหาร มหาวิทยาลัยเกษตรศาสตร์ 0-2942-8629 ต่อ 626, 908 ผลการสืบค้ นเรื่ องเต็มจากชื่อเรื่ องหรื อบทคัดย่อ (Title or Abstract) เรื่ อง กวาวเครื อขาว (Pueraria mirifica) มีทงสิ ั ้ ้น 20 เรื่ อง ดังนี ้ 1. CA1199276 - 1/14/1986 HAIR COSMETIC PREPARATION URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=CA1199276 Inventor(s): KRIWET MANFRED (--) Applicant(s): KRIWET MANFRED (--) IP Class 4 Digits: A61K; A61Q IP Class:A61K8/00; A61K8/97; A61Q5/00; A61Q5/02; A61Q7/00; A61K8/96; A61K7/06 Application Number: CA19820408408 (19820729) Priority Number: DE19813130894 (19810731) Family: CA1199276 Equivalent: WO8300433; EP0084546; SU1205748; FI831047; DE3130894; BR8207791; IT1198404; FI71224C; FI71224B; AT15759T; AU553366B2; AU8738382A; BR8207791A; CA1199276A1; DE3130894A1; DE3130894C2; DK100183A; DK100183D0; DK163710B; DK163710C; EP0084546A1; EP0084546B1; FI831047A; FI831047D0; IT1198404B; IT8222578D0; JP4019962B; JP58501175T; MX162474A; NO163804B; NO163804C; NO830675A; SU1205748A3; WO8300433A1; ZA8205284A Abstract: Abstract of CA1199276 Hair cosmetic preparation, such as hair tonic, restorer, shampoo or the like based on conventional constituents containing an extract of olive stones, pomegranate pips, seeds of the fruit of Glossostemon bruguieri, and tubers of Pueraria mirifica. The aforementioned extracts are obtained by conventional extraction using suitable extractants. The hair cosmetic preparations according to the invention have a marked hair growth-aiding action. A hair tonic preferably comprises 10 to 25% by volume of the extract in fluid form. Mathéo Software (c) http://www.matheo-software.com 1/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 2. CN1483734 - 3/24/2004 NOVEL PROCESS FOR EXTRACTING PUERARIN FROM PUERARIA MIRIFICA URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=CN1483734 Inventor(s): ZHUO YUZHE (CN) Applicant(s): ZHUO YUZHE (CN) IP Class 4 Digits: A23K; C07H IP Class:A23K1/00; C07H1/08; C07H17/07; C07H1/00; C07H17/00 Application Number: CN20031034472 (20030726) Priority Number: CN20031034472 (20030726) Family: CN1483734 Equivalent: CN1483734A Abstract: Abstract of CN1483734 The technological process for extracting puerarin from wild pueraria root includes the following steps: cold-pressing and pulverizing raw material, reflux extraction by using ethyl alcohol at 59-78 deg.C, evaporation and concentration, recovering ethyl alcohol, separation and fermentation so as to obtain the invented product whose pueraria content is greater than 93.8%. At the same time the residual impurity produced by extraction of puerarin can be fermented by adding yeast, and can be made into multivitamin feed. Mathéo Software (c) http://www.matheo-software.com 2/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 3. DE3130895 - 2/10/1983 FEED OR FEED ADDITIVE FOR ANIMALS URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=DE3130895 Inventor(s): KRIWET MANFRED (DE) Applicant(s): JUNGE HANSA IMPORT EXPORT GMBH (DE) IP Class 4 Digits: A23K IP Class:A23K1/14; A23K1/16 Application Number: DE19813130895 (19810801) Priority Number: DE19813130895 (19810801) Family: DE3130895 Equivalent: DE3130895A1; DE3130895C2 Abstract: Abstract of DE3130895 Feed or feed additives for animals, which are characterised in that they are composed of an extract of olive stones, apple pips, Glossostemon bruguieri, tubers of Pueraria mirifica and/or corn cobs or ground olive stones and/or apple pips. The ground olive stones and/or apple pips are produced by conventional grinding. The extracts mentioned are likewise obtained by conventional extraction using suitable extractants. The feeds or feed additives according to the invention are highly suitable for processing in mixed feeds.Description: Gegenstand der Erfindung sind Futtermittel bzw. Futter mittelzusätze für Tiere und deren Verwendung in Mischfuttermitteln. Seit vielen Jahren werden von der Futtermittelindustrie verstärkte Anstrengungen unternommen, Futtermittel bzw. Futtermittelzusätze zu entwickeln, die einen positiven Einfluss auf das Wachstum von Tieren haben. Die meisten der heutzutage auf dem Markt befindlichen sogenannten Wachstums förderer werden auf synthetischem Wege hergestellt und sind dementsprechend verhältnismässig teuer. Es wurde nun überraschend gefunden, dass wachstums fördernde Futtermittel bzw. Futtermittelzusätze in einfacher Weise aus natürlichen Rohstoffen gewonnen werden können. Gegenstand der Erfindung sind dementsprechend Futtermittel bzw. Futtermittelzusätze für Tiere der im Hauptanspruch gekennzeichnete Art sowie deren Verwendung als Bestandteil von Mischfuttermitteln. Die Herstellung der erfindungsgemäss zuverwendenden Extrakte erfolgt durch übliche Extraktion von Olivenkernen, Apfelkernen, insbesondere Granatapfelsamen (Punica granatum), Glossostemon bruguieri, Knollen der Pueraria mirifica (einer Leguminoseart) oder Maiskernen. Dabei werden z.B. entfettete oder nicht entfettete Olivenkerne mit geeigneten organischen Lösungsmitteln wie Aceton, Methanol, n-Propanol, Isopropanol, Eisessig oder Gemischen derselben ausgezogen und daraus FluidExtrakte, Spissum Extrakte oder Trockenextrakte hergestellt. Üblicherweise werden die beispielsweise eingesetzten Olivenkerne vor der Extraktion nach herkömmlichen Verfahren gemahlen. Mathéo Software (c) http://www.matheo-software.com 3/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Insbesondere bei der erfindungsgemässen Verwendung von Oliven- und Apfelkernen kann jedoch auf die Extraktion verzichtet werden und die gemahlenen Oliven- und Apfelkerne können bereits als solche als Futtermittel bzw. Futtermittel zusatz verwendet werden. Die erfindungsgemässen Futtermittel und Futtermittel zusätze eignen sich insbesondere für die Fütterung von Schweinen, Rindern und Geflügel, können aber auch zur Fütterung anderer Tiere verwendet werden. Überraschenderweise ist bei Verfütterung der erfindungsgemässen Futtermittel bzw. Futtermittel zusätze eine deutliche Steierung des Wachstums zu beobachten. So wurde bei Versuchen mit Mäusen festgestellt, dass die Gewichtszunahme bei denjenigen Tieren, deren Futter gemahlene Olivenkerne bzw. Olivenkernextrakt enthielt, über der von anderen Tieren lag, die mit dem gleichen Futter ohne Zusatz von gemahlenen Olivenkernen bzw. Olivenkernextrakt ernährt wurden. Es ist offensichtlich, dass die erfindungsgemässen Futtermittel bzw. Futtermittelzusätze im Falle der Verwendung von Extrakten vorwiegend in Form der Spissum-Extrakte oder insbesondere der Trockenextrakte verwendet werden, da sich diese am besten zur Weiterverarbeitung in Mischfuttermitteln eignen. Bei geeigneter Auswahl des Extraktionsmittels und bei speziellen Fütterungsarten können jedoch auch Fluid-Extrakte verwendet werden. Zur Extraktion sei bemerkt, dass diese im allgemeinen mit ausreichend Extraktionsmittel durchgeführt wird, so dass eine erschöpfende Extraktion erzielt wird. Selbstverständlich können auch Mischungen der verschiedenen zuvor genannten Extrakte bzw. Mischungen dieser Extrakte mit gemahlenen Oliven- und/oder Apfelkernen verwendet werden.Data supplied from the esp@cenet database - Worldwide Claims: Futtermittel bzw. Futtermittelzusatz für Tiere Patentansprüche 1. Futtermittel bzw. Futtermittel zusatz für Tiere be stehend aus einem Extrakt von Olivenkernen, Apfel kernen, Glossostemon bruguieri, Knollen der Pueraria mirifica und/oder Maiskernen oder aus gemahlenen Oliven-und/oder Apfelkernen besteht. 2. Verwendung des Futtermittels bzw. Futtermittel zusatzes nach Anspruch 1 als Bestandteil von Mischmuttermitteln.Data supplied from the esp@cenet database - Worldwide Mathéo Software (c) http://www.matheo-software.com 4/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 4. EP1242103 - 9/25/2002 EXTRACTS DERIVED FROM PUERARIA MIRIFICA, BUTEA SUPERBA AND/OR MUCUNA COLLETTII AND EXTRACTION THEREOF URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=EP1242103 Inventor(s): CHERDSHEWASART WICHAI (TH) Applicant(s): CHEIL JEDANG CORP (KR); CHERDSHEWASART WICHAI (TH) IP Class 4 Digits: A61K; A61Q; A23L; A61P IP Class:A61K8/00; A61K8/97; A61K8/96; A23L2/38; A23L1/212; A23L1/29; A23L1/30; A23L1/304; A23L2/52; A61K8/11; A61K8/19; A61K9/20; A61K9/48; A61K36/00; A61K36/48; A61K47/02; A61P3/00; A61P3/06; A61P9/10; A61P13/08; A61P15/00; A61P15/10; A61P15/12; A61P17/00; A61P43/00; A61Q19/00; A61K36/185; A61P9/00; A61P13/00; A61K35/78 Application Number: EP20000958984 (20000828) Priority Number: WO2000KR00961 (20000828); TH19990052443 (19990827) Family: EP1242103 Equivalent: WO0115718; US6673377; JP2004115521; EP1242103; AU7038600A; EP1242103A1; EP1242103A4; JP3502374B2; JP2003508041T; JP2004115521A; KR20010025178A; US6673377B1; US7060305B2; US2004105900A1; WO0115718A1 Cited Document(s): GB785987; GB453583; XP002234082; CN1144123; XP002234083; CN1196256; XP001097251; XP009005287; XP001122010; XP009005273; XP001097250; JP6345634; XP002234084; JP6080553 Abstract: Abstract not available for EP1242103 Abstract of corresponding document: WO0115718 The present invention relates to extracts derived from Pueraria mirifica, Butea superba and/or Mucuna collettii and extraction thereof, foods, beverages, pharmaceutical products and/or cosmetics containing the extracts as an active ingredient and manufacturing thereof. The extracts isolated from the said plants contain higher concentration of isoflavones. The products produced from composition containing the extracts considerably increase a resilience and gloss of skin at its application in human body.Description: EXTRACTS DERIVED FROM PUERARIA MIRIFICA, BUTEA SUPERBA AND/OR MUCUNA COLLETTII AND EXTRACTION THEREOF TECHNICAL FIELD The present invention relates to extracts derived from Pueraria mirifica (white Kwao krua), Butea superba (red Kwao Krua) and/or Mucuna collettii (black Kwao Krua), an extraction process therefor, foods, beverages, pharmaceutical products and/or cosmetics containing the extracts as active ingredients, and a process for manufacturing the same. BACKGROUND ART Pueraria mirifica, a leguminous plant found mainly in Thailand, was introduced to the West in the 1930s and has been used as a Thai folk medicine for invigorating sexual activities (Nature, December 3,1960,774). Particularly, its tubers have long been used for invigorating post-menopausal females. Mathéo Software (c) http://www.matheo-software.com 5/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Butea superba is also a leguminous plant found mainly in Thailand. This plant provides red sap and its tubers have long been used as a raw material for invigorating males in Thailand. Mucuna collettii is a tall leguminous plant found mainly in the subtropical zones. When being cut and exposed to the air, all parts of this plant turn black. Likewise, its roots have long been used as a folk medicine for invigorating males. In those plants are found 25 or more compounds, including miroesterol, diadzin, diadzein, genistin, genistein, beta-sitosterol, stigrmsterol, mirificoumestan, kwarkhurin, mirificine, long chain alcohols, and dioxyisoflavone. Particularly, those plants are found to contain phytoestrogen in far larger quantities than do other leguminous plants. Isoflavones are distributed in a variety of plants and extensively found particularly in such leguminous plants as soybean, arrowroot and alfafa. Isoflavones are termed phytoesterogens on the grounds that not only do these compounds have structures similar to those of endogenous estrogens, but also their physiological actions bring about the same effects as those of estrogens. Phytoestrogens, however, show insignificant physiological activity compared with estrogens. Where phytoestrogens are ingested for a long period of time, it is reported that estrogen hormone-like functions are exerted in the body (Bingham SA, Atkinson C, J. Nutr 1988 79 (5) 393-406). In terms of subgroups, phytoestrogens may be broken down into cou-mestrol, genistgein,formonetin, diadzein, biochanin A, most of which are based on isoflavone. There was reported one interesting study concerning phytoestrogen, in which attention was paid to the fact that Japanese women suffer from less serious menopausal diseases than do Western women. This has been revealed to be attributed to the diet of the Japanese, who consume soybean related foods containing much of phytoestrogen. Now, the use of phytoestrogen in overcoming menopausal disease is now under extensive study. Genistein, a phytoestrogenic material which can be extracted from leguminous plants, for example, is prescribed to overcome a menopausal disease. This compound is also used to prevent breast cancer or cardiovascular diseases. Additionally, diosgenin, a precursor to progesterone which is responsible for the menstrual cycle, is now commercially available as a cream formulation while being extracted from Mexican wild yams. There have successively been reported noteworthy research results on the effects of phytoestrogen on the reduction of breast cancer attack rate. In the case of Asian women, they are attacked by breast cancer, ovarian cancer, and uterine cancer less than women of the other regions since they have long consumed foods prepared from leguminous plants, which contain much phytoestrogen (Adlercreutz H, Honjo H., Am. J. Clin. Nutri. 1991,54 (6), 109 1100). Furthermore, phytoestrogen is known to be of anti-oxidative activity as well as being active in preventing or improving osteoporosis (Tsutsmi N, Biol. Pharm. bull 1995,18 (7) 1012-1015). In vivo, phytoestrogen binds to an estrogen receptor, showing an estrogen agonist or antagonist activity. The level at which estrogen is produced in vivo can be elucidated with the affinity for the binder. At the time of menopause, because estrogen is produced in a very small quantity, phytoestrogen's action may be relatively increased. Generally, phytoestrogen has a hormonal activity which is nothing but 1/400 to 1/1000 that of estrodiol's. However, this low activity has the advantage of showing no side effects that estrogen has. Hence, when having long been ingested, phytoestrogen is recognized as preventing the side effects estrogen can bring about, as demonstrated in various research results. Despite many research results, the accurate therapeutic mechanism and effects associated with phytoestrogen have not yet been discovered. Mathéo Software (c) http://www.matheo-software.com 6/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) One of the findings disclosed as to phytoestrogen, thus far, is the physiological activity upon application to the skin. While estrogen was applied to the skin of menopausal females, who are generally in rapid progression of dermal aging, it was observed that their skin was inhibited from progressing into senility. In detail, after 6 months of the application of estrogen, the skin noticeably became resilient with a 60 % improvement in the depth of wrinkles. This effect was reported to be attributed to an increase in the number of dermal collagen fibers (Schmidt JB, Binder M., Int. J.Dermatol., 1996,35 (9), 669-674). Composing the hypoderm, elastin tissues form a network, along with collagen tissues, in the skin. Elastase, a protease which hydrolyses elastin, is known to play an important role in the antiinflammatory mechanism. This enzyme attacks all proteins which act to maintain the backbone and shape of the connective tissues, such as elastin, collagen, proteoglycan and keratin, nonselectively as well as indiscriminately. Collagenase is known to exert its catalytic action on a limited number of substrates (Wiedow,O., Schroder, J. M., E. J. Biol. Chem. 265 (25), 14791 (1990)). Exposure to UV-A makes the skin suffer repeatedly from mild inflammation in response of the catalytic action of elastase on reticular tissues and brings about damage in elastin and collagen fibers, finally making the skin sag (Motoyoshi, K., Tacenouch, M., Proceedings of the19'h IFSCC Congress Sydney 22-25, October 1996). Beginning in their forties, people generally have their skin rapidly decrease in resilience. From a biochemical point of view, as people grow older, elastase is increasingly active in the body, which results in destroying or aggregating a part of the elastin fibers and losing of collagen fibers (Bissett, D. L., Photochem. Photobiol., 1987,46,367 378). Recently, extensive research has been directed to the finding of inhibitory materials against elastase activity. For instance, cosmetics for topical application are commercially available, which contain elastase inhibitory materials in order to prevent the skin from being xeric owing to UV irritation or to inhibit the skin from progressing into senility. Particularly, plant extracts with anti-oxidation activity are under the intensive study on the retardation of dermal aging or wrinkling because they have been discovered to inhibit activity of elastase (Bizot Foulon V., Godeat G., W. Int. J. Cos. Sci. 17,225-264 (1995)). DISCLOSURE OF THE INVENTION With the knowledge that Pueraria mirifica, Butea superba or Mucuna collettii, all of which have estradiol activity 40 to 100 times as great as that of any other leguminous plant, e. g., containing phytoestrogen at a level of approximately 0.51. U. on average, is intimately associated with collagen, the present inventor primarily tested them for their effect on the stratum corneum in vitro. Typically, most leguminous plants range, in phytoestrogen activity, from about1/20,000 to1/50 1. U. (1I. U. = 0.1 mg of estrodiol). Mica, Butea superba and Mucuna collettii showed high inhibitory activity against elastase as expected on the basis that each of them is relatively high in phytoestrogen activity (about 0.5I. U.). Leading to the present invention, the intensive and thorough research conducted by the present inventor, resulted in the finding that extracts derived from Pueraria mirifica, Butea superba and Mucuna collettii are not high in anti oxidation activity, but highly inhibitory against the activity of elastase and that they have various applications in the cosmetic, pharmaceutical, and food industry. Therefore, it is an object of the present invention to provide an extract derived from Pueraria mirifica, Butea superba, and/or Mucuna collettii. It is another object of the present invention to provide a method for extracting pharmaceutically effective ingredient form Pueraria mirifica, Butea superba and/or Mucuna collettii. Mathéo Software (c) http://www.matheo-software.com 7/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) It is a further object of the present invention to provide foods, beverages, medicines and/or cosmetics, which contain as an effective ingredient an extract derived from Pueraria mirifica, Butea superba and/or Mucuna collettii. It is still another object of the present invention to provide a method for preparing foods, beverages, medicines and/or cosmetics, which contains as an effective ingredient an extract derived from Pueraria mirifica, Butea superba and/or Mucuna collettii. To achieve one of the above objects, there is provided an extract derived from Pueraria mirifica, Butea superba and/or Mucuna collettii, wherein tubers, roots, stems, leaves and/or tissue-cultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii are subjected to chemical extraction using water, organic solvents or mixtures thereof as an extractant. To achieve one of the above objects, there is provided a method for preparing an extract from Pueraria mirifica, Butea superba and/or Mucuna collettii, in which tubers, roots, stems, leavesand/or tissuecultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii are dried in a temperature and time-controllable oven, pulverized, and immersed in water, an organic solvent or a mixture thereof, and the resulting solution was spray-, freeze and/or vacuum-dried. To achieve one of the above objects, there is provide a method for preparing food products, beverage products, pharmaceutical products and/or cosmetic products, comprising the steps of : extracting pharmaceutically effective ingredients from Pueraria mirifica, Butea superba and/or Mucuna collettii by drying tubers, roots, stems, leaves and/or tissue-cultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii in a temperature and timecontrollable oven, pulverizing them into pieces or powders, immersing the plant pieces or powders in water, a low alcohol with one or more hydroxyl groups, or a mixture thereof, and optionally drying the solution in a spray-drying, a freezedrying and/or a vacuumdrying manner; and using the extract alone as a sole material or combining the extract with a base, a diluent, an additive, a dye, an active agent, a surfactant, a wetting agent, an anti-oxidant and/or other additives suitable for use in foods, beverages, pharmaceutical products and/or cosmetics at an amount of 0.1-99.9% by weight or volume based on the total weight or volume. Herein, the products comprise the dried extract or the liquid extract at an amount of 0.1-100 % and are preferably in the form selected from the group consisting of pills, capsules, packages, bottles, and boxes or in any other sealed form. Advantageously, the extract is further added with inorganic calcium or organic calcium. Preferably, the drying process is carried out at40-90 C for 2-9 hours in an oven. To achieve one of the above objects, there is provided use of the fine powder, dry extract, extract and/or active ingredients derived from Pueraria mirifica, Butea superba and/or Mucuna collettii as a raw material for manufacturing cosmetics, functional cosmetics, medicinal cosmetics and pharmaceutical products suitable for use in skin care, breast care, breast firmness, breast enlargement, wrinkle removal from the breast; healthy aid foods, functional foods, beverages and pharmaceutical products suitable for use in the treatment of prostate hyperplasia; foods, beverages and pharmaceutical products suitable for use in the prevention of hypercholesterolemia and arteriosclerosis; foods, beverages and pharmaceutical products suitable for use in the treatment of erection dysfunction or malfunction; and/or foods, beverages and pharmaceutical products suitable for use in the treatment of menopausal and postmenopausal symptoms. BEST MODES FORCARRYING OUT THE INVENTION A better understanding of the present invention may be obtained in light of the following examples which are set forth to illustrate, but are not to be construed to limit the present invention. EXAMPLE1 Preparation of Pueraria mirifica Extract Mathéo Software (c) http://www.matheo-software.com 8/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 100 g of the tuber of Pueraria mirifica was cleansed, dried at70 C for about 5 hours by use of a hot-air drier, and pulverized. To the resulting plant pieces was added 500 mL of a mixture of methanol and water (80/20 v/v), followed by the extraction at50 C for about 6 hours. After being filtered, the extract was concentrated in a vacuum to remove the solvent. The concentrate was freeze-dried leaving a brownish solid which was then measured for isoflavone content. Contents of isoflavones in the extract derived from the tuber of Pueraria mirifica are given in Table1, below. TABLE 1 Contents of Isoflavones in Extract Derived from Tuber of Pueraria mirifica Isoflavone Content(mg/100g) Daidzin 51. 0 Daidzein 8.1 Genistin 12.0 Genistein 2.0 Puerarin 96.0 As shown in Table 1, the above processes can prepare the best quality of Pueraria mirifica powder. The powder prepared according to the above processes was measured to be high in its contents of isoflavone and other phytoestrogens. Phytoestrogens obtained from the plant included miroesterol, daidzin, genistin, genistein, betasitosterol, stigmasterol, coumestrol, puerarin, campesterol, mirificoumestan, kwakhurin, and mirificin (Bounds and Pope, 1960; Ingham et al., 1986a; Ingham et al., 1986b; Ingham et al., 1988). Isoflavone has potent anti-cancer activity particularly against breast cancer, rectal cancer and prostatic cancer. High contents of isoflavones in the Pueraria mirifica prepared in the present invention are very beneficial to the human body. Chemical extraction of high contents of isoflavone profiles from Pueraria mirifica can be achieved by slicing all plant parts into pieces or grinding the plant to powder and immersing the pieces or powder in a solvent, such as water, a low molecular weight alcohol which contains one or more hydroxyl groups, and that prepared by a synthetic technique. The extract may be used as it is or may be further purified or concentrated for use. EXAMPLE 2 Skin Lotion Containing Extract Derived From Pueraria mirifica A cosmetic composition for skin lotion was prepared, which contained the Pueraria mirifica extract at an amount of 4.0 % by weight, butylenes glycol at an amount of 4.0 % by weight, sodium ethylenediaminetetraacetate at an amount of 0.001 % by weight, citric acid at an amount of 0.01 % by weight, ethanol at an amount of 15.0 % by weight, paraoxybenzoic acid methyl at an amount of 0.1 % by weight, polyoxyethylenesorbitan monostearate at an amount of 0.5 % by weight, perfume at an amount of 0.2 % by weight, and water at an amount of the remaining to 100 % by weight. To this end, the emulsion ingredients and the aqueous ingredients were dissolved in separate baths, and then emulsified in the emulsion bath, together, followed by aging them at30 C. TABLE 2 Composition of Skin Lotion Containing Pueraria mirifica Extract Composition Content (wt%) Pueraria mirifica Extract 4.0 Butyleneglycol 4.0 EDTA-Na 0.001 Citric Acid 0.01 Ethanol 15.0 Paraoxybenzoic acid methyl 0.1 Polyoxyethylene sorbitanmonostearate 0.5 Perfume 0.2 Purified Water to 100 EXAMPLE 3 Mathéo Software (c) http://www.matheo-software.com 9/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Lotion Containing Extract Derived From Pueraria mirifica Using the composition shown in Table 3, below, a lotion containing an extract derived from Pueraria mirifica was prepared in the same manner as in Example 2. TABLE 3 Composition of a Lotion Containing a Pueraria mirifica Extract Composition Content (wt%) Pueraria mirifica Extract 4.0 Cetostearyl Alcohol 1.0 Glyceryl Monostearate 0.8 Sorbitan Monostearate 0.3 Propyl Paraben 0.1 Polysorbate 60 1.0 Mineral Oil 5.0 Cyclomethicone 3.0 Dimethicone 0.5 Allantoin 0.1 Glycerin 5.0 Propyleneglycol 3.0 Methyl Paraben 0.2 Triethanol Amine 0.2 Carbomer 8.0 Purified Water To 100 EXAMPLE 4 Essence Lotion Containing Extract Derived From Pueraria mirifica Using the composition shown in Table 4, below, an essence lotion containing an extract derived from Pueraria mirifica was prepared in the same manner as in Example 2. TABLE 4 Composition of Essence Lotion Containing Pueraria mirifica Extract Composition Content (wt %) Pueraria mirifica Extract 6.0 Butylene Glycol 4.0 Glycerin 3.0 Milk Extract 1.0 Allantoin 0.1 Pantenol 0.1 EDTA-Na 0.01 Carbomer 0.15 Aqueous Collagen Sol'n 0.5 Ethanol 6.0 Polyoxyethylene Octyldodecyl Ether 0.3 Parabenzoic Acid Methyl 0.1 Triethanol Amine 0.15 Purified Water To 100 EXAMPLE 5 Cream Containing Extract Derived From Pueraria mirifica Using the composition shown in Table 5, below, a cream containing an extract derived from Pueraria mirifica was prepared in the same manner as in Example 2. TABLE 5 Composition of Cream Containing Pueraria mirifica Extract Composition Content (wt %) Pueraria mirifica Extract 4.0 Cetostearyl Alcohol 1.0 Glyceryl Monostearate 1.0 Sorbitan Monostearate 0.3 Microcrystalline Lead 0.2 Mathéo Software (c) http://www.matheo-software.com 10/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Propyl Paraben 0.1 Petrolatum 1.0 Polysorbate 60 1.0 Mineral Oil 5.0 Cyclomethicone 3.0 Dimethicone 0.5 Avocardo Oil 2.0 Allantoin 0.1 Glycerin 5.0 Propylene Glycol 3.0 Methyl Paraben 0.2 Triethanol Amine 0.2 Carbomer 17.2 Xanthan Gum 0.1 Purified Water To 100 TEST EXAMPLE1 Application of Cosmetic Composition to the Body The cream containing the extract from Pueraria mirifica, prepared in Example 5, was applied to the body and tested for improvement in skin resilience and gloss. In this regard, 44 healthy female volunteers in their twenties to fifties were selected: for 39 women, a cream containing the Pueraria mirifica extract was used: for 4 women, a placebo cream which contained no Pueraria mirifica extracts was used. The cream was applied uniformly to the whole area of the breast twice a day, preferably in the morning and evening, with a daily dosage of 2 g for 2 months. On the3t'-5"day after menstruation, the first application of the cream was conducted. At that time, the breast was measured for resilience. After two months of the application, a measurement was made of the resilience of the breasts of the subjects. As for breast resilience, its measurement was conducted on an area 1 cm distant from the center of a nipple in the direction of 12 o'clock, with the aid of Cutometer SEM474 (Courge+Khazaka Electronic GmbH). The results are given in Tables 6 and 7, below. TABLE 6 Change in Breast Resilience Between Pre-and Post-Application of Cosmetic Composition of the Invention Treatment Group Pt. No Pre-Application (mm)Post-Application (mm) 1 0. 645 0.968 2 0.824 0.878 3 0.737 0.923 4 0.969 1 5 0.917 0.935 6 0.875 0.923 7 0.97 0.905 8 0.976 0.951 9 0.951 0.878 10 0.897 1.048 11 0.947 0.939 12 0. 906 0.952 13 0. 931 0.966 14 0. 938 0.94415 0. 926 1 16 0. 895 0.824 17 0. 794 0.888 18 0. 895 0.895 19 0. 889 0.9444 20 0. 933 0.974 21 0. 938 0.88922 0. 8 0.82 23 0. 889 0.81 24 0. 967 0.965 25 0. 929 0.923 26 0. 931 0.939 27 1 0.882 28 0. 875 0.8429 0. 903 0.951 30 0. 964 1 31 0. 967 0.970 32 0. 84 0.941 33 0. 811 0.74234 0. 869 1 35 0. 821 0.824 36 0. 517 0.889 37 0. 771 0.967 38 0. 880 0.968 39 0. 875 0.882 Control Grouppt.N. Pre-Application (mm) Post-Application (mm) 1 0. 95 0. 9 2 0.794 0.95 0.05 at a one-sided test. That is, the null hypothesis was accepted and no improvements were brought about in breast resilience. In addition to breast resilience, the cream was tested for breast enlargement. In this regard, the effect of the extract from Pueraria mirifica on breast enlargement was determined by measuring the volumes of the breast before and after the application of the cream in an MRI machine. No remarkable breast enlargement was found from the statistics. Mathéo Software (c) http://www.matheo-software.com 11/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) TEST EXAMPLE 2 Application as Food Aid or Functional Food to Pre-Menopausal Female Extracts derived from Pueraria mirifica according to the present invention were used as food aids or functional foods and consumed by 97 healthy women who were pre-menopausal and 20-45 years old, as shown in Table 8, below. TABLE 8 Medicinal Efficacy of Extract from Pueraria mirifica as Food Aid or Functional Food Placebo 400 mg/day800 mg/day No. ofSubjects 31 32 34 Breast Pain 31 14 32 Smooth Skin 31 28 32 Hair Complexity 31 24 30 Charasma Clearness-3/3 4/4 Smooth Vaginal Secretion 31 14 29 Smooth Menstruation 31 2 10 Breast Resilience 31 13 30 Breast Enlargement 31 3 28 Hip Enlargement 31 3 20 31 subjects in a placebo group were each administered 2 tapioca starch capsules a day every other day for 2 months from the first day of their menstruation. All parameters measured in this placebo group were shown not to undergo a significant change. 32 subjects in a first treatment group were each administered 2 Pueraria mirifica capsules, each having 200 mg, per day in the same conditions as in the placebo group. Significant improvements were brought about in various parameters, including breast or bust pain (44 %) which partially resulted from breast or bust firmness and enlargement, smooth skin (88 %), healthy hair (75 %), charasma clearness (100 %), vaginal secretion (44 %), smooth menstruation (6 %), breast or bust firmness (44 %) and enlargement (9 %), and hip enlargement (9 %). 33 subjects in the second treatment group were each administered 4 Pueraria mirifica capsules, each having 200 mg, per day in the same conditions as in the placebo group. After 2 months of the administration, greater improvements were found in all of the parameters measured, including breast or bust pain (94 %), healthy hair (88 %), charasma clearness (100 %), smooth vaginal secretion (85 %), breast or bust firmness (94 %) and enlargement (88 %), and hip enlargement (59 %). In some subjects, blood cholesterol levels were observed to decrease after the administration. Consequently, food aids or functional foods comprising the extract derived from Pueraria mirifica can increase feminine sex appeal through improvement in skin condition, hair complexity, and breast firmness and enlargement. TEST EXAMPLE 3 Application of Food Aid or Functional Food Containing Pueraria mirifica Extract to Post-Menopausal Female The extract derived from Pueraria mirifica in Example 1 was used as a food aid or prepared into a functional food. Six women were each administered one capsule containing 200 mg of the Pueraria mirifica extract a day for 21 days of one month over four months. The subjects were 35-58 years old and suffered from fever, slightly mild delusion, xeroderma,and/or disease of menstruation or menopause. Their health was checked with blood examinations, just before and after the administration. The results are given in Table 9, below. TABLE 9 Mathéo Software (c) http://www.matheo-software.com 12/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Effect of Food Aid or Functional Food containing Pueraria mirifica Extract on Post-Menopausal Women Pre-Consumption Post-Consumption Subject No. Age Symptom Menstruation Symptom Menstruation1 46 Fever, Delusion Paused Normal Paused 2 52 Fever, Delusion Paused Normal Paused 3 35 Xeroderma Ceased Normal Resumed 4 58 Fever Paused Normal Paused 5 49 Fever Continued Normal Continued 6 39 Delusion Almost paused Normal Normal As apparent from Table 9, the extract from Pueraria mirifica can be useful as a food aid or a functional food with excellent therapeutic effects on menopausal symptoms such as fever, mild delusion, xeroderma, and irregular menstruation. TEST EXAMPLE 4 Cutaneous Allergy Assay of Pueraria mirifica Extract The extract derived from Pueraria mirifica in Example 1 was assayed as to whether it causes cutaneous allergies. The assay was conducted on six Winstar rats and six rabbits. The results are given in Table 10, below. TABLE 10 Cutaneous Allergy Assay of Pueraria mirifica Extract on Winstar Rats and Rabbits Animal Winstar Rat Rabbit 1'Response No Allergic Response No Allergic Response 2'Response No Allergic Response No Allergic Response From the results inducing no allergic response from the test animals, the extract derived from Pueraria mirifica according to the present invention is expected to also be safe to human skin. EXAMPLE 6 Preparation of Butea superba Extract 100 g of the tuber of Pueraria mirifica was cleansed, dried at70 C for about 5 hours by use of a hot-air drier, and pulverized. To the resulting plant pieces was added 50 mL of a mixture of methanol and water (80/20 v/v), followed by the extraction at50 C for about 6 hours. After being filtered, the extract was concentrated in a vacuum to remove the solvent. The concentrate was freezedried leaving a brownish solid. TEST EXAMPLE 5 Effect of Butea superba as Food Aid or Functional Food In order to determine whether the extract prepared in Example 6 could be used as a food aid or a functional food, 142 mal patients with erection dysfunction, who were 20-55 years old, were orally administered the extract or a placebo. The subjects in the placebo groups ingested a placebo capsule containing 200 mg of tapioca starch twice a day, e. g., after breakfast and supper. The impotence patient in a first treatment group were administered one capsule containing 200 mg of the Butea superba extract twice a day, e. g., after breakfast and supper, for two months while the patients in a second treatment group was administered two capsules, each containing 200 mg of the Butea superba extract, twice a day after breakfast and supper for the same period of time. Eight parameters related to erection dysfunction were measured and the results are given in Table 11, below. TABLE11 Effect of Butea superba on Male with Erection Dysfunction Upon Oral Administration Placebo400 mg/day 800 mg/day No. of Subjects 42 46 1st1stErection Day after Administration 42 4.160.81 (MeanS. D) Immediate Erection 42 32 43 (+4) Mathéo Software (c) http://www.matheo-software.com 13/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Continuous Erection 42 28 42 (+4) erection Maintenance for 2 min after 29(+4)4214 Ejaculation Weekly Frequency of Erection 42 32 43(+4) No. of Intercou comprising both Butea superba and Mucuna collettii are provided, which are useful in the treatment of erection dysfunction or malfunction. Consequently, the extract derived from Butea superba according to the present invention is therapeutically effective for male sexual activity. This plant is believed to have vasodilation activity in the penis. Through this activity, Butea superba may be used directly or as an effective ingredient for the treatment of erection dysfunction. To demonstrate this hypothesis, 65 males with erection dysfunction or malfunction were divided into two groups. The subjects in one group were allowed to consume a placebo capsule containing 200 mg of tapioca starch twice a day, e. g., after breakfast and supper. On the other hand, a capsule containing 200 mg of Butea superba was administered to the subjects of the other group, twice a day after breakfast and supper. All of them were measured for erectile function. The results are given in Table 12, below. TABLE 12 Effect of Butea superba on Erection Dysfunction or Malfunction Total No of Subjects No. of Restored Males Placebo 31 2 Treatment 34 28 As seen in Table 12, only a few patients could have erectile ability in the placebo group while most subjects in the treatment group restored the sexual potency. Additionally, after being administered the extract from Butea superba, none of the subjects suffered from prostate hyperplasia. This result was attributed to the fact that the plant containedss-sitosterol, which is reported to act as a potent preventive against prostate hyperplasia (Klipel et al. 1997). With such evidence, Butea superba can be also prepared into a therapeutic medicine for preventing prostate hyperplasia or a health aid food. In order to obtain more potent therapeutic effects, Butea superba may be combined with Mucuna collettii. Accordingly, a medicine based on Butea superba is provided for the treatment of erection dysfunction or malfunction. TEST EXAMPLE 6 Cutaneous Allergy Assay of Butea superba Extract The extract derived from Butea superba in Example 6 was assayed as to whether it causes cutaneous allergies. The assay was conducted on six Winstar rats. The results are given in Table 13, below. TABLE 13 Cutaneous Allergy Assay of Butea superba Extract on Winstar Rats 1'Allergic Response No 2'Allergic Response No From the results shown in Table 13, it is recognized that the extract derived from Butea superba according to the present invention can be safely applied to human skin or developed into cosmetics or medicines for topical use. TEST EXAMPLE 7 Effect of Gel Made of Butea superba Extract on Erection Dysfunction 63 males were divided into two groups for testing the Butea superba extract for effectiveness as a therapeutic medicine for erection dysfunction. For the placebo group, gel containing no Butea superba was applied to the penis twice a day for one month. On the other hand, the same procedure was conducted for the treatment group, except that gel containing Butea superba was used. After observation, the results are given in Table 14, below. Mathéo Software (c) http://www.matheo-software.com 14/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) TABLE 14 Effect of Butea superba on Erection Dysfunction Upon Topical Application Total No. of Subjects No. of Restored Male Placebo 31 Treatment 32 21 The restoration in the placebo group is believed to result from a psychological effect or a massage effect during the application of the gel. In contrast to those in the placebo group, may of the subjects in the treatment group could restore their sexual ability. In consequence, products for topical use, which is effective for the treatment of erection dysfunction or malfunction, can be prepared from Butea superba. In order to prepare an extract powder from Mucuna collettii, the same process as those applied to Pueraria mirifica or Butea superba may be used. The extract powder from Mucuna collettii is of highly potent vasodilation activity without causing serious side effects. Chemical extraction from these three herbs Pueraria mirifica, Butea superba and Mucuna collettii can be achieved by slicing into pieces all plant parts, including roots, tubers, stems, leaves, and calluses grown through tissue culture, and immersing the pieces or powder in a solvent, such as water, a low molecular weight alcohol which contains one or more hydroxyl groups, and that prepared by a synthetic technique. The extract may be homogeneously mixed with appropriate bases to produce cosmetics or pharmaceutical medicines for topical use. Novel mixtures were also found to exhibit transdermal penetration activity. In addition, extracts were derived from the three herbs under a high hydraulic pressure. They are also easily mixed with other ingredients suitable for use in the preparation of beverages. From Pueraria mirifica, Butea superba and Mucuna collettii, powders of pharmaceutically effective ingredients were prepared by a spray drying, a freeze drying or a vacuum drying process. Any of the processes is suitable to obtain high quality of extracts. In the present invention, pharmaceutically effective ingredients can be effectively extracted from the three herbs by chemical extraction and obtained as a concentrate by distillation. The extract thus obtained according to this method exhibits high medicinal efficacy. INDUSTRIAL APPLICABILITY As described hereinbefore, the extracts derived from Pueraria mirifica, Butea superba and/or Mucuna collettii according to the present invention are high in their contents of isoflavones. When being applied to the human body, the cosmetics, pharmaceutical products and/or foods prepared from the extracts can bring about a great improvement in skin resilience and gloss and be useful for the treatment of erection dysfunction or malfunction. Therefore, they can find various applications in the food industry, the beverage industry, the pharmaceutical industry and the cosmetic industry.Data supplied from the esp@cenet database - Worldwide Claims: CLAIMS 1. An extract derived from Pueraria mirifica, Butea superba and/or Mucuna collettii, wherein tubers, roots, stems, leaves and/or tissue-cultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii are subjected to chemical extraction using water, organic solvents or mixtures thereof as an extractant. 2. A method for preparing an extract from Pueraria mirifica, Butea superba and/or Mucuna collettii, in which tubers, roots, stems, leaves and/or tissuecultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii are dried in a temperature and time-controllable oven, pulverized, and immersed in water, an organic solvent or a mixture thereof, and the resulting solution was spray-, freeze-and/or vacuum-dried. 3. A method for manufacturing foods, beverages, pharmaceutical products Mathéo Software (c) http://www.matheo-software.com 15/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) and/or cosmetics, comprising the stepsof : extracting pharmaceutically effective ingredients from Pueraria mirifica, Butea superba and/or Mucuna collettii by drying tubers, roots, stems, leaves and/or tissue-cultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii in a temperature and time-controllable oven, pulverizing them into pieces or powders, immersing the plant pieces or powders in water, a low alcohol with one or more hydroxyl groups, or a mixture thereof, and optionally drying the solution in a spray-drying, a freeze-drying and/or a vacuum-drying manner; and using the extract alone as a sole material or combining the extract with a base, a diluent, an additive, a dye, an active agent, a surfactant, a wetting agent, an anti oxidant and/or other additives suitable for use in foods, beverages, pharmaceutical products and/or cosmetics at an amount of 0.1-99.9 % by weight or volume based on the total weight or volume of the food, beverage, pharmaceutical product or cosmetic. 4. The method as set forth in claim 3, wherein said products comprise the dried extract or the liquid extract at an amount of 0.1-100 % and are in the form selected from the group consisting of pills, capsules, packages, bottles, and boxes or in any other sealed form. 5. The method as set forth in claim 3, wherein the extract is further added with inorganic calcium or organic calcium. 6. A method for manufacturing foods, beverages, pharmaceutical products and/or cosmetics, in which tubers, roots, stems, leaves and/or tissue-cultured calluses of Pueraria mirifica, Butea superba and/or Mucuna collettii are dried at40-90 C for 2-9 hours in an oven, pulverized into pieces or powders, and immersed in an extractant selected from water, a low alcohol with one or more hydroxyl groups, and a mixture thereof, the solution was dried in a spray-drying, a freeze-drying and/or a vacuum-drying manner to give an extract, and the extract is combined at a volume or weight ratio of 0.1: 99.9 to 100: 0 with an additive selected from the group consisting of a base, a diluent, a dye, an active agent, a surfactant, a wetting agent, an anti-oxidant, other agents suitable for use in the foods, beverages, pharmaceutical products and/or cosmetics, and mixtures thereof. 7. Use of the fine powder, dry extract, extract and/or active ingredients derived from Pueraria mirifica, Butea superba and/or Mucuna collettii as a raw material for manufacturing cosmetics, functional cosmetics, medicinal cosmetics and pharmaceutical products suitable for use in skin care, breast care, breast firmness, breast enlargement, wrinkle removal from the breast; healthy aid foods, functional foods, beverages and pharmaceutical products suitable for use in the treatment of prostate hyperplasia; foods, beverages and pharmaceutical products suitable for use in the prevention of hypercholesterolemia and arteriosclerosis; foods, beverages and pharmaceutical products suitable for use in the treatment of erection dysfunction or malfunction; and/or foods, beverages and pharmaceutical products suitable for use in the treatment of menopausal and postmenopausal symptoms.Data supplied from the esp@cenet database - Worldwide Mathéo Software (c) http://www.matheo-software.com 16/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 5. GB785987 - 11/6/1957 IMPROVEMENTS IN OR RELATING TO OESTROGENIC SUBSTANCES URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=GB785987 Inventor(s): POPE GERALD SEATON (--) Applicant(s): NAT RES DEV (--) IP Class 4 Digits: None IP Class:None Application Number: GB19550010796 (19550414) Priority Number: GB19550010796 (19550414) Family: GB785987 Equivalent: GB785987A Abstract: Abstract of GB785987 An oestrogen having the empirical formula C20H22O and designated "miroestrol" is prepared by subjecting a methanol or ethanol extract of tuberous roots of pueraria mirifica to partition chromotography. The oestrogen reduces Fehling's solution, is destroyed by alkali, gives an anhydroderivative C19H20O5 with hydrochloric acid, gives a monomethyl ether, melts with decomposition at 270 DEG C. and has an optical rotation of [a ]17 DEG = + 301 DEG . Powdered roots of pueraria mirifica are first extracted with aqueous methanol or ethanol then with pure methanol or ethanol and the combined extracts evaporated to dryness. The residue is taken up with methanol and after standing, the granular precipitate is filtered off and the filtrate evaporated to dryness. This residue is dissolved in methanol and ether is added with the formation, on standing, or a gummy precipitate. The supernatant solution is decanted, evaporated to dryness and the residue again treated with methanol and ether as before. The precipitate which formed is dissolved in methanol and re-precipitated with ether and the combined supernatant solutions evaporated to dryness. Ethyl acetate was added and the solution left to clarify, the filtrate was evaporated to dryness and partitioned between benzene, ethyl acetate, methanol and water. The lower phase was evaporated to dryness and the residual chromotographed on a large Kieselguhr column using benzene and ethyl acetate as the mobile phase and methanol and water as the stationary phase. Aliquots of the various fractions were examined by paper chromatography and an oestrogen-rich fraction rechromatographed on a smaller column. After two such treatments, the fractions containing the oestrogen partially crystallized and the oestrogen was re-crystallized from methanol. Specifications 137,051, 453,583, [both in Group VI], and U.S.A. Specification 2,112,712 are referred to.ALSO:A process for the production of an oestrogen comprises subjecting a methanol or ethanol extract of tuberous roots of pueraria mirifica to partition chromatography. It was formerly thought that the roots producing the oestrogen were of the species butea superba, and an oestrogen has been described by Butenardt of empirical formula C19H20O6 isolated from these roots. In the Specification the oestrogen is designated "miroestrol" with an empirical formula C20H22O6 but is said to be identical with that described by Butenhardt. Powdered roots of pueraria mirifica are first extracted with aqueous methanol or ethanol, then with pure methanol or ethanol and the combined extracts evaporated to dryness. The residue is taken up with methanol and after standing the granular precipitate is filtered off and the filtrate evaporated to dryness. This residue is dissolved in methanol and ether is added with the formation, on standing, of a gummy precipitate. The supernatant solution is decanted, evaporated to dryness and the residue again treated with methanol and ether as before. The precipitate which forms is dissolved in methanol and reprecipitated with ether and the combined supernatant solutions evaporated to dryness. Ethyl acetate was added and the solution left to clarify, the filtrate was Mathéo Software (c) http://www.matheo-software.com 17/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) evaporated to dryness and partitioned between benzene, ethyl acetate, methanol and water. The lower phase was evaporated to dryness and the residue chromatographed on a large Kieselguhr column using benzene and ethyl acetate as the mobile phase and methanol and water as the stationary phase. Aliquats of the various fractions were examined by paper chromatography and the oestrogen-rich fractions rechromatographed on a smaller column. After two such treatments the fractions, containing the oestrogen, partially crystallized and the oestrogen was recrystallized from methanol, melting with decomposition at 270 DEG C. Specifications 437,051, 453,583, and U.S.A. Specification 2,112,712 are referred to.Description: PATENT SPECIFICATION Inventor: GERALD SEATON POPE Date of filing Complete Specification March 26, 1956. Application Date April 14, 1955. 785,987 No 10796/55. Complete Specification Published Nov 6, 1957. Index at Acceptance:-Classes 2 ( 3), C 3 A 11; and 81 ( 1), B 12 B. International Classification: -A 61 k, C 07 g. COMPLETE SPECIFICATION Improvements in or relating to Oestrogenic Substances We, NATIONAL RESEAR Cii DEVELOPMENT CORPORATION, a British Corporation, of 1, Tilney Street, London, W 1, do hereby declare the invention, for which we pray that a patent may be granted to us, and the method by which it is to be performed, to be particularly described in and by the following statement: This invention relates to the isolation of naturally-occurring oestogenic substances and is particularly concerned with the isolation of an oestrogen from a Siamese plant which was formerly incorporated in a Siamese rejuvenating drug. This drug, which was reported in 1932 and 1933 to have been used in Siam, consisted of the tuberous roots of a plant, powdered and mixed with honey and myrobalans It was reported to have brought on menstruation in old women, to have enabled an impotent old man to become the father of new offspring and also in some cases to have been toxic The tuberous root which had these effects was thought to be that of Butea superba, Roxb. Schering-Kahlbaum obtained supplies of the roots and in Specifications Nos 437,051 and 453,583 they describe extraction methods using water, acetone, chloroform and methanol All the extracts were highly oestrogenic in the rat but that prepared with water was the most potent The specification also described various ways of separating the oestrogen from inactive matter as follows:(a) The roots were extracted with hot water; the extract filtered and the filtrate evaporated to a syrup, which was then diluted with a large excess of methanol and filtered The filtrate possessed oestrogenic activity Repetition of this process concentrated the active matter further. (b) The active matter from (a) obtained by evaporating the filtrate to dryness was re-dissolved in water and precipitated with ammonium sulphate Oestrogenic activity was found in the precipitate. (c) Dried powdered roots were extracted lPrice 3 s 6 d l with methanol The methanol solution was evaporated; the residue dissolved in water and precipitated with ammonium sulphate The precipitate was partitioned between benzene 50 and 70 % ethanol and the oestrogenic activity was then found mainly in the lower layer. (d) The precipitated matter from (b) possessing activity was re-dissolved in methanol and ether added to precipitate impurities; this 55 being repeated twice, the oestrogen remaining largely in solution. Mathéo Software (c) http://www.matheo-software.com 18/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) (e) The active matter from (d) obtained by evaporating the solution to dryness was dissolved in ethanol and water added until the 60 solution contained 70 % of ethanol This was then extracted with benzene The oestrogen remained in the aqueous ethanol. Schoeller, Dohrn and Hohlweg (United States Specification No 2,112,712) again 65 found the roots to be rich in oestrogen (highly active orally as well as subcutaneously) They extracted the dried, powdered roots with ethanol and then filtered and evaporated the solution They note the isolation of an oestro 70 gen of the empirical formula GIH 22,0. Later these workers published their work in short form (Naturwissenschaften 28, 532 ( 1940)) They described the acquisition of the oestrogenic roots from Siam, which they call 75 butea superba, but they noted that the oestrogenic plant appeared to be different from the butea superba growing in botanical gardens in Ceylon They estimated the oestrogenic activity of the roots to be 180,000 Rat Units 80 per kilogram or equivalent to 150 mg of oestrone per kg They prepared highly oestrogenic concentrates from which Butenandt and Jacobi at Danzig, first isolated the oestrogen and the gave the empirical formula C 1,l H,0 06 85 (Butenandt, Naturwissenschaften 28, 533 ( 1940)) Butenandt found that the compound reduced Fehiing's solution, was destroyed by alkali, gave an anhydro derivative CJH 1 OO, with HC 1 and gave a monomethyl ether 90 Schoeller, Dohrn and Hohlweg (Naturwissenschaften 28, 532 ( 1940)) also described 785,987 the various physiological effects on the rat of the new compound These are typical of stimulation by oestrogen The new substance lay between oestradiol and oestrone in activity when assayed by the subcutaneous route in the Ailen-Doisy test; it was much more active orally than either oestradiol or oestrone. It was finally established that the plant producing the tuberous roots containing the oestrogen is of a new species which is named pueraria miriica, Airy Shaw et Suvatabandhu (Kew Bulletin ( 1952, 549)). It is an object of this invention to provide an improved process for the isolation of the aforesaid oestrogen to which we have ascribed the empirical formula C 20 H 2206, but which is presumably identical with that described by Butenandt to which he gave the empirical formula G 10 H 20 G O This compound will hereinafter be referred to as "miroestrol " Particulars of the ultra-violet and infra-red absorption spectra are given below in Example 1. According to the process of the present invention for the production of miroestrol, a methanol or ethanol extract of tuberous root of pueraria mirifica is subjected to partition chromatography. Two methods were used for assaying the various extracts and fractions for oestrogen content; bioassay by the mouse uterine weight assay as used by Pope & Roy (Biochem J 53, 427 ( 1953)) and paper chromatographic assay. Bioassay which can estimate the total oestrogenic activity of a crude extract was used during the development of the isolation process but once miroestrol had been concentrated sufficiently it was possible and convenient to estimate it by paper chramatography. According to a preferred embodiment of the invention powdered tuberous roots of pueraria mirifica are extracted with aqueous methanol or ethanol and then with pure methanol or ethanol and the solutions separated, for example by filtration, combined together and evaporated to dryness The residue from this is fractionated by solution in methanol and ether added to precipitate impurities Most of the oestrogen remains in solution and is further purified by partition in a solvent system containing water, methanol, ethyl acetate and benzene The active material remains in the lower phase and is next subjected to partition chromatography. Preferably the partition chromatography is carried out on a column of kieselguhr and preferably also the mobile phase is a mixture of benzene and ethyl acetate and the stationary phase is aqueous methanol. The following examples illustrate the invention:1 The initial material is tuberous roots of pueraria mirifica sliced, sun-dried and powdered to a fine powder in a hammer-mill and having a dry matter content of approximately 87 %. Mathéo Software (c) http://www.matheo-software.com 19/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) kg of powder was boiled and stirred for 0.5 hour with a mixture of 11 litres of methanol and 1 litre of water in a 20 litre flask, provided with a reflux condenser and a stirrer consisting of a stainless steel shaft and six 70 small vanes set at an angle of 450 to the shaft. The stirrer was driven by a 0 08 H P electric motor at 1350 r p m by direct coupling with rubber tubing The solution was separated by filtration through an 8-inch Buchner funnel 75 having a tin-plate, cylindrical extension piece to increase its capacity, the filtrate passing directly into a 20 litre distillation flask The plant residue was then similarly extracted a second time with 10 litres of methanol and a 80 third time also with 10 litres of methanol The combined filtrates were evaporated to dryness under reduced pressure, the residue shaken with 3 litres of methanol and left to stand for 18 hours after which the granular precipitate 85 was filtered off and the filtrate evaporated to dryness at reduced pressure The residue was then dissolved in 1 liitre of methanol and 4 litres of ether added On leaving overnight a gummy precipitate formed from which the 90 supernatant solution was decanted This solution was then evaporated to dryness and the residue dissolvel in 1 litre of methanol and 4 litres of ether added Again after allowing to stand the clear supernatant solution was 95 decanted off, the precipitate being redissolved in 500 ml of methanol and re-precipitated with 2 litres of ether The combined supernatant solutions were then evaported to dryness at reduced pressure This residue was shaken 100 with 2 5 litres of ethyl acetate at room temperature and the solution left to clarify The filtrate was evaporated to dryness and partitioned between: Benzene 2500 ml 1 ^ 5 Ethyl acetate 250 ml Methanol 1250 nl Water 1250 ml The lower phase was separated off and the upper phase washed with 1250 ml of 50 % 110 aqueous methanol The combined lower phases were evaporated to dryness at reduced pressure and the residue chromatographed on a large column using the partition method The solvent system used was: 115 Benzene 8000 ml Ethyl acetate 3000 ml Methanol 5000 ml Water 5000 ml The column consisted of a 6 ft length of 120 glass pipeline of 4 inches internal diameter with a suitable reduction piece and stop-cock at its lower end It was packed with kieselguhr (Celite 545, supplied by Johns-Manville Co, Ltd, London, the word " Celite " being a 125 registered Trade Mark) by the method of Martin (Biochem Soc Symposia No 3, p 11, 1949) The kieselguhr was slurried with solvent by mechanical stirring in a flask and the slurry transferred by pressure into the top of 130 -785,987 the column Packing was done with a stainless steel ramrod having a perforated head ( 8 inch diameter holes) and a sectional shaft The height of the kieselguhr was then 28 inches. The residue to be chromatographed was added in the minimum quantities of a mixture of stationary and mobile phases necessary to effect solution The partition column was operated in the usual manner and suitable aliquots of the various fractions examined by paper chromatography The chromatograms were sprayed with a suitable visualising agent such as diazotised p-aminophenyl-2-diethylaminoethyl sulphone, methanolic potassium hydroxide or periodic acid. The fractions from the 4-inch diameter column containing miroestrol were then rechromatographed on a 2-inch diameter kieselguhr column using the same solvent system. 2 C The fractions containing miroestrol were again identified by paper chromatography and were collected together and run similarly on a second 2-inch column The fractions from this column which contained miroesterol crystallised partially and were washed with ethyl acetate yielding crystals ( 140 mg) Recrystallisation from methanol gave pure miroestrol ( 120 mg), m.p (with decomposition) 2700 C, optical rotation lOlC'7 D = + 3010. The ultra-violet absorption of miroesterol in ethanol solution was as follows: ethanol A max. ethanol A 220 my; 287 my e= 3480 max. The infra-red absorption spectrum of miroesterol (crystalline state; as paraffin paste) showed absorption peaks at: 3334 cm -', 2865 cm -', 1712 cm -', 1664 cm.', 1621 cm ', 1597 cm -', 1508-1504 cm.1, 1460-1453 cm -', 1401 cm ', 1362 cm?, 1325 cm -1, 1282 cm -', 1245-1242 cm, 12,24-1218 cm -', 1183 cm -', 1176 cm.?', 1170 cm -', 1160 cm -, 1155 cm -'. The oestrogenic activity of miroestrol was about 1 3 times that of oestradiol-17/3 in the mouse uterine weight test and about one-fourth that of oestradiol-17/ in the rat vaginal smear test (Allen & Doisy, Amer J Physiol 69, 577 ( 1924)). Mathéo Software (c) http://www.matheo-software.com 20/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) It was estimated by mouse bioassay methods that the overall recovery of miroestrol from the plant source was greater than 50 %. 2 Example 1 was repeated using an equal volume of ethanol instead of methanol for the two extractions of the plant.Data supplied from the esp@cenet database - Worldwide Claims: What we claim is: 1 A process for the production of miroestrol as herein defined wherein a methanol or ethanol extract of tuberous roots of pueraria mirifica is subjected to partition chromatography. 2 A process for the production of a miroestrol wherein powdered tuberous roots of p'ueraria mirifica are extracted with aqueous methanol or ethanol, the extract is separated from the roots and then the extracted roots are extracted with pure methanol or ethanol, the extract is separated from the roots, the extracts are combined together and evaporated to dryness, the residue is fractionated by solution in methanol and addition of ether to precipitate impurities, the residue obtained by evaporation to dryness of the supernatant solution is purified by partition in a solvent system containing water, methanol, ethyl acetate and benzene and the lower phase is subjected to partition chromatography. 3 A process as claimed in Claim 1 or 2 wherein the partition chromatography is carried out on a column of kieselguhr. 4 A process as claimed in Claim 3 wherein the mobile phase is a mixture of benzene and ethyl acetate and the stationary phase is aqueous methanol. A process for the production of miroestrol substantially as described with reference to either of the examples. 6 Miroestrol when produced by the process claimed in any one of the preceding claims. ELKINGTON & FIFE, Consulting Chemists and Chartered Patent Agents, Bank Chambers, 329, High Holborn, London, W C 1, Agents for the Applicants. PROVISIONAL SPECIFICATION Improvements in or relating to Oestrogenic Substances We, NATIONAL RESEARCH DEVELOPMENT CORPORATION, a British Corporation, of 1, Tilney Street, London, W 1, do hereby declare this invention to be described in the following statement: This invention relates to the isolation of naturally-occurring oestrogenic substances and is particularly concerned with the isolation of an oestrogen from a Siamese plant which was formerly incorporated in a Siamese rejuvenating drug. This drug, which was reported in 1932 and 1933 to have been used in Siam, consisted of 105 the tuberous roots of a plant, powdered and mixed with honey and myrobalans It was reported to have brought on menstruation in old women, to have enabled an impotent old man to become the father of a new offspring 110 and also in some cases to have been toxic The tuberous root which had these effects was thought to be that of Butea superba, Roxb. Scherin-Kahlbaum obtained supplies of the 785,987 roots and in French Specification No 782,375 they describe extraction methods using water, acetone, chloroform and methanol All the extracts were highly oestrogenic in the rat but that prepared with water was the most potent. The specification also describes various ways of separating the oestrogen from inactive matter as follows:(a) The roots were extracted with hot water; the extract filtered and the filtrate evaporated to a syrup, which was then diluted with a large excess of methanol and filtered The filtrate contained the activity Repetition of this process concentrated the activity further. (b) The active matter from (a) was re-dissolved in water and precipitated with ammonium sulphate The activity was found in the precipitate. Mathéo Software (c) http://www.matheo-software.com 21/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) (c) Dried powdered roots were extracted with methanol The methanol solution was evaporated; the residue dissolved in water and precipitated with ammonium sulphate The precipitate was partitioned between benzene and 70 % ethanol and the oestrogenic activity was then found mainly in the lower layer. (d) The precipitated matter from (b) containing activity was re-dissolved in methanol and precipitated with ether; this being repeated twice, the oestrogen remaining largely in solution. (e) The active matter from (d) was dissolved in ethanol and water added until the solution contained 70 % of ethanol This was then extracted with benzene The oestrogen remained in the aqueous ethanol. Schoeller, Dohrn and Hohlweg (United States Specif Ication No 2,112,712) again found the roots to be rich in oestrogen (highly active orally as well as subcutaneously) They extracted the dried, powdered roots with ethanol and then filtered and evaporated the solution They note the isolation of an oestrogen of the empirical formula CQH 22-0. Later these workers published their work in short form (Naturwissenschaften 28, 532 ( 1940)) They described the acquisition of the oestrogenic roots from Siam, which they call butea superba, but they noted that the oestrogenic plant appeared to be different from the butea superba growing in botanical gardens in Ceylon They estimated the oestrogenic activity of the roots to be 180,000 Rat Units per kilogram or equivalent to 150 mg of oestrone per kg They prepared highly oestrogenic concentrates which were given to Butenandt and Jacobi at Danzig, who first isolated the oestrogen and gave the empirical formula CQH,2 00 (Butenandt, Naturwissenschaften 28, 533 ( 1940)) Butenandt found that the compound reduced Fehliing's solution, was destroyed by alkali, gave an anhydro derivative CQH,0, with HGL and gave a monomethyl ether. Schoeller, Dohrn and Hohlweg (Naturwissenschaften 28, 532 ( 1940)) also described the various physiological effects on the rat of the new compound These are typical of stimulation by oestrogen The new substance lay between oestradiol and oestrone in activity when assayed by the subcutaneous route in the Allen-Doisy test; it was much more active 70 orally than either oestradiol or oestrone. It was finally established that the plant producing the tuberous roots containing the oestrogen is of a new species which is named pueraiia mirifica, Airy Shaw et Suvatabandhu 75 (Kew Bulletin ( 1952, 549)). It is an object of this invention to provide an improved process for the isolation of the aforesaid oestrogen which has been found to have the empirical formula CQH-0,0 but 80 which is apparently identical with the compound described by Butenandt to which he gave the empirical formula CQH 2,J 06 This compound will hereinafter be referred to as "miroestrol " 85 According to the process of the present invention for the production of miroestrol, a methanol extract of tuberous roots of pueraria mirifica is subjected to partition chromatography 90 According to a preferred embodiment of the invention powdered roots of piel-aria minifica are extracted with methanol and then with aqueous methanol and the solution separated by filtration and evaporated to dryness 95 The residue from this is fractionated by solution in methanol and precipitation with ether. Most of the oestrogen remains in solution and is further purified by partition in the solvent system water, methanol, ethyl acetate, benzene 100 The active material remains in the lower phase and is next subjected to partition chromatography. Preferably the partition chromatography is carried out on a column of kieselguhr and pre 105 ferably also the mobile phase is a mixture of benzene and ethyl acetate and the stationary phase is aqueous methanol. The following example illustrates the invention: 110 The initial material was powdered roots of puraria mirifica having a dry matter content of 87 %. 500 g of powdered roots was boiled for 0 5 hour with 2 5 litres of methanol and the solu 115 tion separated by filtration The powder was then re-extracted with 2 5 litres of boiling methanol-water ( 80:20 v/v) and this solution separated by filtration The latter process was then repeated The combined solutions were 120 then evaporated to dryness at reduced pressure and the residue mixed with 500 ml Mathéo Software (c) http://www.matheo-software.com 22/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) of methanol The process was then repeated on a second 500 g quantity and the two final 500 ml methanol solutions combined, filtered and 125 the filtrate evaporated at reduced pressure to 500 ml To this 500 ml of ether was added slowly with shaking A gummy precipitate formed from which the solution was decanted The solution was 130 -4 785,987 evaporated to dryness and the residue remaining dissolved in methanol ( 120 ml) and shaken while ether ( 500 ml) was added Again the precipitate and solution were separated by decantation This precipitate was re-treated by dissolving in methanol ( 100 ml) and reprecipitating with ether ( 300 ml) The two solutions were then combined, evaporated to dryness and treated with ethyl acetate ( 500 ml) The solution was evaporated to dryness and the residue equilibrated in the solvents benzene ( 500 ml), ethyl acetate ( 50 ml), methanol ( 250 ml) and water ( 250 ml) The upper phase of this was extracted with fresh lower phase ( 250 ml) and the two lower phase solutions combined and evaporated to dryness The resulting final extract was found to contain much of the original oestrogenic activity. Another 5000 g of the initial material was then processed similarly and all the final extracts (from 6000 g) bulked This extract, about 30 g in all, had approximately 50 % of the original oestrogenic activity, or about 100 mg of oestrogen expressed as miroestrol. The 30 g of extract was then divided into eight equal portions Each portion was chromatographed on a column using the partition method The column consisted of a vertical glass tube 5 cm in diameter and 100 cm. long with a glass tap at its lower end A plug of glass wool was first pressed tightly into the column which was then packed with kieselguhr (" Celite 545 ") in the manner described by Martin (Biochem Soc Symposia No 3, p. 11, 1949) using the system benzene ( 800 ml), ethyl acetate ( 300 ml), methanol ( 500 ml), water ( 500 ml) The top phase was then run through the column until its top surface was level with the top of the packing The column of kieselguhr (from 400 g dry weight) when completed was 62 cm tall The material to be chromatographed (approximately 3 6 g) was then dissolved in 2 to 3 ml of lower phase and the solution placed on to the surface of the packing Elution with mobile phase was then begun and the following volumes of elute collected in successive fractions: 1000, 500, 250, 250, 250, 250, 500, 600 ml Finally residual plant extract was removed from the column by eluting with methanol Data for a typical chromatogram are in Table I. TABLE I 3.7 G EXTRACT (DERIVED FROM 652 G DRY TUBEROUS ROOT) MATTER OF PUERARIA No of fraction Volume of successive Wts of plant Oestrogenic activity fractions eluted (ml) extract in calculated as fraction (mg) miroesterol (mg) 1000 500 250 250 250 250 500 600 methanol eluate 1662 344 59 56 42 59 1309 3641 nil nil nil 0.15 2.5 ; 8 ; 2 nil nil ; 12 65 These results show that the activity after one chromatographic process has been concentrated into approximately 150 mg of material ( 25-fold concentration) Activity recovered is at least 50 % of that originally in the plant material. Active fractions from all eight chromatograms, on which were processed the extract from 6000 g of initial material, were pooled and re-processed through two more chromatograms Finally 82 mg of crystalline matter, sparingly soluble in ethyl acetate, was obtained. Recrystallisation of this yielded 47 5 mg of microscopic, colourless, rod-like crystals, m p. (with decomposition) 2750 C, which were characterised as miroestrol by paper chromatography using three solvent systems. The product has the empirical formula C 19 H 220 H 2 O. The substance was soluble in alcohols but very sparingly soluble in ether, ethyl acetate, benzene or chloroform It formed a penta-acetate CQH,,O ( O Ac), It had activity equal to 90 that of oestradiol in the mouse uterine weight test and one-fourth that of oestradiol in the Allen-Doisy test in the rat, when given subcutaneously in each case. The oestrogenic activity of extracts was 95 estimated by the mouse uterine weight method. Mathéo Software (c) http://www.matheo-software.com 23/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) No attempt was made to use the method in its statistically valid form although at least two dose levels of each unknown extract were used and the responses compared with those given 100 by known quantities of the standard oestrogen, oestradiol This enabled estimates of the oestrogenic activity of extracts to be made that were in practice sufficiently accurate to enable the work of isolation of the oestrogen to pro 105 CHROMATOGRAM OF 1 2 3 4 6 7 8 -5 785,987 ceed smoothly A description of the bioassay as used in this way is given by Pope and Roy, Biochem J 53, 427 ( 1953). ELKINGTON & FIFE, Consulting Chemists and Chartered Patent Agents, Bank Chambers, 329, High Holborn, London, W C 1, Agents for the Applicants. Leamington Spa: Printed for Her Majesty's Stationery Office, by the Courier Press,-1957. Published at the Patent Office, 25, Southampton Buildings, London, W C 2, from which copies may be obtained.Data supplied from the esp@cenet database - Worldwide Mathéo Software (c) http://www.matheo-software.com 24/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 6. JP2000316526 - 11/21/2000 MEDICAL HERB CONTAINING FOOD COMPOSITION URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=JP2000316526 Inventor(s): SUPOI SANRITTOWANICHA (--) Applicant(s): BIO PURE CO LTD (--) IP Class 4 Digits: A23L IP Class:A23L1/30 Application Number: JP19990123571 (19990430) Priority Number: JP19990123571 (19990430) Family: JP2000316526 Equivalent: JP2000316526A Abstract: Abstract of JP2000316526 PROBLEM TO BE SOLVED: To provide a food composition which is safe and good for health, and a method for preparing the composition. SOLUTION: This food composition comprises four kinds of medicinal herbs of pueraria mirifica, boesenbergia pandurata, eugenia caryophllus and glycyrrhiza glabra, and lactose and/or gelatin as a powdery filler; and the manufacturing process contains a formulation step for granules or tablets. Mathéo Software (c) http://www.matheo-software.com 25/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 7. JP2001220340 - 8/14/2001 SKIN PREPARATION FOR EXTERNAL USE FOR PREVENTING AGING URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=JP2001220340 Inventor(s): (--) HOSHINO HIROSHI (--); HIGUCHI YOSHIHIRO (--); MORIYAMA MASAHIRO Applicant(s): KOSE CORP (--); SHIRATORI PHARM (--) IP Class 4 Digits: A61K; A61Q; A61P IP Class:A61K8/00; A61K8/97; A61K8/96; A61K8/19; A61K36/48; A61P17/00; A61Q19/00; A61K36/185; A61K35/78; A61K8/29; A61K8/30; A61K8/98; A61Q1/00; A61Q1/12; A61K7/48; A61K8/33; A61K8/34; A61K8/49; A61K8/72; A61K8/73; A61K8/99; A61Q19/08; A61K7/00 Application Number: JP20000029348 (20000207) Priority Number: JP20000029348 (20000207) Family: JP2001181170 Equivalent: CN1301543A; JP2001181170A; JP2001220340A; KR20010057585A; US6352685B2; US2001014311A1 Abstract: Abstract of JP2001220340 PROBLEM TO BE SOLVED: To provide a skin preparation for external use for preventing aging by compounding a newly found component having excellent preventing activities against aging such as effective improvement of wrinkles or slackness on the skin, the preventing activities being higher than conventional cell-activating agent. SOLUTION: A skin preparation for external use for preventing aging, composed of an extract of Pueraria mirifica as a preventive component against aging. Further, a skin preparation for external use for preventing aging, composed of the extract and a pharmaceutically effective component selected from a cell activating agent, an antioxidant, a humectant and a UVproofing agent. Mathéo Software (c) http://www.matheo-software.com 26/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 8. JP2001352939 - 12/25/2001 SUPEROXIDE SCAVENGING ACTIVATOR URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=JP2001352939 Inventor(s): ONISHI SAYAKA (--) Applicant(s): SANYU CO LTD (--) IP Class 4 Digits: A61K; A23L; A61P IP Class:A23L1/212; A23L1/30; A61K36/48; A61K36/185; A61K35/78; A61P39/06; A61P39/00 Application Number: JP20000221530 (20000615) Priority Number: JP20000221530 (20000615) Family: JP2001352939 Equivalent: JP2001352939A Abstract: Abstract of JP2001352939 PROBLEM TO BE SOLVED: To obtain a powder product prepared by drying a specific part of subterranean stem of Pueraria mirifica having superoxide scavenging activity, being noticed as a regulatory substance of excessive active oxygen considered to participate in an outbreak of lifestylerelated diseases. SOLUTION: This dried powder product having superoxide scavenging activity is characterized by thinly shaving a part to 3 cm from the peel of subterranean stem of Pueraria mirifica and drying and powdering the part. Mathéo Software (c) http://www.matheo-software.com 27/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 9. JP2004131500 - 4/30/2004 SKIN AGING INHIBITOR OR IMPROVER URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=JP2004131500 Inventor(s): SAKURAI TETSUTO (--) Applicant(s): FANCL CORP (--); ATTENIR CORP (--) IP Class 4 Digits: A61K; A61Q; A61P IP Class:A61K8/00; A61K8/97; A61Q5/00; A61K8/96; A61K8/11; A61K36/00; A61P17/00; A61P43/00; A61Q19/00; A61K35/78; A61K8/30; A61Q1/00; A61Q1/12; A61K7/48; A61K8/49; A61Q19/08; A61K7/00; A61K31/352; A61K31/353; A61P17/16; A61Q1/04; A61Q1/10; A61Q11/00; A61Q1/02 Application Number: JP20030326816 (20030918) Priority Number: JP20030326816 (20030918); JP20020273733 (20020919) Family: JP2004131500 Equivalent: JP2004131500A Abstract: Abstract of JP2004131500 PROBLEM TO BE SOLVED: To provide a skin aging inhibitor or improver having an excellent effect of inhibiting or improving the skin aging caused by ultraviolet rays, and to provide a cosmetic containing the same. SOLUTION: This skin aging inhibitor or improver contains (a) one, two or more kinds of extracts from plants containing isoflavone or its glycoside and (b) one, two or more kinds of extracts selected from a group comprising extracts from plants containing apigenin or its glycoside and/or plants containing procyanidin. The cosmetic contains the inhibitor or improver. An extract from soybeans, alfalfa leaves, red clover flowers, pueraria mirifica roots or white lupine seeds is exemplified as the extract from the plant containing the isoflavone or its glycoside. An extract from grapefruit seeds, perilla seeds or chamomile flowers is exemplified as the extract from the plant containing the apigenin or its glycoside. An extract from grape, apple, strawberry or cranberry fruits or seeds thereof is exemplified as the extract from the plant containing the procyanidin. COPYRIGHT: (C)2004,JPO Mathéo Software (c) http://www.matheo-software.com 28/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 10. JP2005263696 - 9/29/2005 EPILATION PREVENTIVE URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=JP2005263696 Inventor(s): YAMASHITA HIROMICHI (--); WAKAGUWA TAKEO (--) Applicant(s): YAMASHITA HIROMICHI (--); WAKAGUWA TAKEO (--) IP Class 4 Digits: A61K; A61Q; A61P IP Class:A61K8/00; A61K8/97; A61Q5/00; A61Q7/00; A61K8/96; A61K7/06; A61K36/00; A61K36/48; A61P17/00; A61K36/185; A61K35/78; A61K36/899; A61P17/14; A61K36/88 Application Number: JP20040079402 (20040319) Priority Number: JP20040079402 (20040319) Family: JP2005263696 Equivalent: JP2005263696A Abstract: Abstract of JP2005263696 PROBLEM TO BE SOLVED: To provide a composition for preventing epilation, having an epilation prevention effect and prepared by causing a specific enzyme liquid to act on a decoction of Pueraria mirifica and Sasa veitchii. SOLUTION: The composition having an epilation prevention effect is yielded by adding a specific enzyme liquid to an extract of Pueraria mirifica and Sasa veitchii to cause the enzyme liquid to act on the extract. Thus yielded composition exhibits a remarkable epilation prevention effect in comparison with an extract of Pueraria mirifica, an extract of Sasa veitchii, and the extract of Pueraria mirifica and Sasa veitchii. A composition yielded by only mixing the extract of Pueraria mirifica and Sasa veitchii with the enzyme liquid does not exhibit an epilation prevention effect; and when only a small amount of the enzyme liquid is added to the extract of Pueraria mirifica and Sasa veitchii, a remarkable epilation prevention effect is not exhibited even after incubation. COPYRIGHT: (C)2005,JPONCIPI Mathéo Software (c) http://www.matheo-software.com 29/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 11. KR20010015935 - 3/5/2001 PUERARIA MIRIFICA EXTRACT AND COMPOSITION OF COSMETIC INCLUDING IT URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=KR20010015935 Inventor(s): WICHAI CHERSHEWASART (--) Applicant(s): WICHAI CHERSHEWASART (--) IP Class 4 Digits: A61K IP Class:A61K7/40 Application Number: KR20000012539 (20000313) Priority Number: KR20000012539 (20000313) Family: KR20010015935 Equivalent: KR20010015935A Abstract: Abstract of KR20010015935 PURPOSE: A pueraria mirifica extract and a composition of cosmetic including it is provided to be excellent of an elastase check activity and increase an elasticity and a gloss of skin by applying the composition of cosmetic to the human body. CONSTITUTION: The process performs hot-air drying a rhizome of a pueraria mirifica, and then pulverizes it. And The process produces an extract extracted to a mixing solvent of methanol/water on a shape of a solid. The process measures a released density of a p-nitroaniline by utilizing a Meo-succinyl-Ala-Ala-Pro-Val-p-nitroanilide. And then the process looks over a checking activity in an elastase of the extracted solid of the pueraria mirifica. The process produces a beauty wash, lotion, essence, and cream containing the pueraria mirifica abstract. The process applies the cream after face washing to thirties-forties 15 women during two months and estimates a degree of an elasticity and a gloss in skin. Mathéo Software (c) http://www.matheo-software.com 30/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 12. KR20030089396 - 11/21/2003 COMPOSITION COMPRISING ALCOHOL EXTRACT OF PUERARIA SP. ROOTS URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=KR20030089396 Inventor(s): BAEK DAE HEON (KR); EOM SU JONG (KR); JUN GUK CHE (KR); NOH YEONG SOE (KR); OH DEOK GEUN (KR); PARK JONG SEOP (KR); PARK MYEONG SUN (KR); PARK SI HO (KR); SHIN HONG SIK (KR) Applicant(s): CHEBIGEN (KR) IP Class 4 Digits: A61K IP Class:A61K35/78 Application Number: KR20020066071 (20021029) Priority Number: KR20020027144 (20020516) Family: KR20030089396 Equivalent: KR20030089396A; KR20050033871A Abstract: Abstract of KR20030089396 PURPOSE: Provided is an anticancer composition comprising the alcohol extract of Pueraria sp. roots, which has excellent anticancer activity with less adverse effect and is thus useful for the prevention and treatment of cancer. CONSTITUTION: An anticancer composition is characterized by containing the alcohol extract of Pueraria sp. roots including Pueraria mirifica and Pueraria lobata. The alcohol extract is selected from PE1, PE2, PE3, PE4, Sub PE-A, Sub PE-B, Sub PE-C, Sub PE-D, Sub PE-E, Sub PEF, Sub PE-G, Sub PE-H, Sub PE-D1, Sub PE-D2, and Sub PE-D3. Mathéo Software (c) http://www.matheo-software.com 31/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 13. KR20030089670 - 11/22/2003 EXTRACTS DERIVED FROM PUERARIA MIRIFICA, BUTEA SUPERBA AND/OR MUCUNA COLLETTII AND EXTRACTION METHOD THEREOF, FOODS, BEVERAGES, PHARMACEUTICAL PRODUCTS AND/OR COSMETICS CONTAINING THE EXTRACTS AS ACTIVE INGREDIENT AND MANUFACTURING METHOD THEREOF URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=KR20030089670 Inventor(s): WICHAI CHERSHEWASART (TH) Applicant(s): CJ CORP (KR); WICHAI CHERSHEWASART (TH) IP Class 4 Digits: A61K IP Class:A61K7/48 Application Number: KR20030076373 (20031030) Priority Number: KR20030076373 (20031030) Family: KR20030089670 Equivalent: KR20030089670A Abstract: Abstract of KR20030089670 PURPOSE: Provided are extracts derived from Pueraria mirifica, Butea superba and/or Mucuna collettii and an extraction method thereof, foods, beverages, pharmaceutical products and/or cosmetics containing the extracts as an active ingredient, and manufacturing method thereof. Therefore, skin elasticity and brilliance are improved. CONSTITUTION: An extract derived from Pueraria mirifica is characterized by extracting callus obtained by the culture of the rhizome, root, stem, or leaf of Pueraria mirifica with a solvent consisting of water and alcohol having at least one hydroxy group. Mathéo Software (c) http://www.matheo-software.com 32/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 14. KR20030089671 - 11/22/2003 EXTRACTS DERIVED FROM PUERARIA MIRIFICA, BUTEA SUPERBA AND/OR MUCUNA COLLETTII AND EXTRACTION METHOD THEREOF, FOODS, BEVERAGES, PHARMACEUTICAL PRODUCTS AND/OR COSMETICS CONTAINING THE EXTRACTS AS AN ACTIVE INGREDIENT AND MANUFACTURING METHOD THEREOF URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=KR20030089671 Inventor(s): WICHAI CHERSHEWASART (TH) Applicant(s): CJ CORP (KR); WICHAI CHERSHEWASART (TH) IP Class 4 Digits: A61K IP Class:A61K35/78 Application Number: KR20030076374 (20031030) Priority Number: KR20030076374 (20031030) Family: KR20030089671 Equivalent: KR20030089671A Abstract: Abstract of KR20030089671 PURPOSE: Provided are extracts derived from Pueraria mirifica, Butea superba and/or Mucuna collettii and an extraction method thereof, foods, beverages, pharmaceutical products and/or cosmetics containing the extracts as an active ingredient and a manufacturing method thereof. The extracts contain high concentration of isoflavones and improve skin elasticity and brilliance. CONSTITUTION: An extract of Butea superba and/or Mucuna collettii is characterized by extracting callus obtained by the culture of the rhizome, root, stem, or leaf of Butea superba and/or Mucuna collettii. A therapeutic composition for impotency contains the extract as an active ingredient. Mathéo Software (c) http://www.matheo-software.com 33/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 15. US2005266970 - 12/1/2005 BREAST ENHANCEMENT SYSTEM URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=US2005266970 Inventor(s): TULLER JEFF (US) Applicant(s): SAVVIER INC (US) IP Class 4 Digits: A63B IP Class:A63B21/008; A63B23/035; A63B21/062; A63B21/02 Application Number: US20050201502 (20050811) Priority Number: US20020245733 (20020917); US19980006692 (19980113); US19960646493 (19960508); US20050201502 (20050811) Family: US2003017920 Equivalent: US6468190; US5735780; US2003017920; US2002160890; US5735780A; US6468190B1; US2002160890A1; US2003017920A1; US2005266970A1 Abstract: Abstract of US2005266970 A chest exerciser includes an elongated plunger housing and a pair of plungers that move in sealed sliding contact with the plunger housing on opposite sides of a common air volume within said plunger housing. An adjustable vent, centrally located between the plungers, communicates with the common air volume. Each plunger is attached to a hand grip. The handles slide on and are coaxial with the plunger housing. In the fully retracted position the grip distance between the proximal end of said first plunger hand grip member and the proximal end of said second plunger hand grip member is between 0 and 10 inches. The exerciser is used in combination with a breast enhancement nutrient, such as pueraria mirifica, which may be applied to the breast as a cream or tonic or ingested as an herb or pill.Description: CROSS-REFERENCE TO RELATED APPLICATION [0001] This application is a continuation-in-part of application Ser. No. 09/006,692 filed on Jan. 13,1998, which is a continuation-in-part of application Ser. No. 08/646,493 filed May 8, 1996, now Pat. No. 6,258,733. BACKGROUND OF THE INVENTION [0002] This invention relates to exercising devices and in particular to a pneumatic resister of linear motion having a selection of handle, attachment and/or anchor means that are positional on opposite ends for direction of selectively push exercise and pull exercise to select muscle groups and to specific muscles in the select muscle groups. [0003] A wide variety of motion resisters have been devised for expending exercise work to build muscles. None are known, however, to be pneumatic motion resisters having equally or selectively variable opposite-directional motion resistance with select handle, attachment and/or anchor means positional on opposite ends in a manner taught by this, invention. [0004] Examples of spring exercise devices that are related but different are described in the following patent documents: U.S. Pat. No. 5,267,929, issued to Chen on Dec. 7, 1993; U.S. Pat. No. 5,246,413, issued to Koblick on Sep. 21, 1993; U.S. Pat. No. 5,026,050, issued to Leung, et al. on Jun. 25, 1991; U.S. Pat. No. 4,483,533, issued to Mangiapane on Nov. 20, 1984; U.S. Pat. No. 3,497,216, issued to Feather on Feb. 24, 1970; and U.S. Pat. No. 2,806,699, issued to Spooner on Sep. 17, 1957. Mathéo Software (c) http://www.matheo-software.com 34/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) Unfortunately, spring-resister type exercise devices are not as safe as pneumatic-resister type exercise devices as skin can get caught between spring coils and the spring device can open quickly and hurt the user. [0005] An example of a different but related pneumatic motion resister is described in U.S. Pat. No. 5,044,630, issued to Ventimiglia on Sep. 3, 1991. The Ventimiglia device, however, was limited to use of elbow pads that specifically direct exercise work to arms instead of to chest and shoulder areas as taught by this invention. Further, the Ventimiglia device was not adaptable to select positioning of handle, attachment and/or anchor means as taught by this invention. [0006] A great need exists for a chest exerciser with which exercise work can be directed precisely to chest muscles. This is important for both women and men. For women, it is because chest muscles make healthy breasts which aid bodily health and do not sag. Instead, muscle at tops and sides of breasts are developed to hold them up, to protect them and to facilitate mammary circulation. Cosmetically, the muscles at tops of breasts cause higher and larger bust configuration. The muscles at sides of breasts add cleavage beauty and breast width that are very attractive. Overall, the breasts can be enlarged with a highly beneficial and safe health effect instead of with breast implants. It also obviates the need for humiliating breast-extension cups. [0007] For men, it is important for some of the same reasons as for women but with different bodystructure effects. Development of chest muscles aids circulation in the chest area and looks good. Areaspecific exercise of chest muscles causes huge male chest buildup that conveys attractive masculinity in a manner in which similar female chest buildup on breasts conveys attractive femininity. [0008] Improved chest circulation for both women and men also aids circulation in arms and hands. Highly important also; it aids vascular circulation for the heart, the head and the nerves of both sexes. [0009] There is an urgently vast need also for area-specificness of a low-weight, small and inexpensive motion resister to be adaptable and adjustable selectively to exercise of other parts of bodies. [0010] Further yet, there is need for motion resistance with area-specificness to be adjustable separately for opposite directions of linear motion resistance. Resistance of both push and pull provides weight-lifting effects in opposite directions. Some muscle groups and some specific muscles in different muscle groups are best exercised with push and others with pull exertion. Particularly chest group muscles of both females and males have some specific muscles that are best exercised with push and others with pull. Arm and leg muscles are similar in relation to different specific muscles. It is direction of appropriate push and pull exertion to specific muscles in muscle groups that gives the special effects of desired muscle protrusion. [0011] Thus a chest exerciser is adaptable to exercise other muscle groups with precise muscle-area specificity that is effective and efficient for chest buildup of both females and males. One of its utilities is its adaptability of appropriate exertion exercise to different muscle groups and to specific muscles in muscle groups throughout a body as well as to the chest area. SUMMARY OF THE INVENTION [0012] In light of need for improved exercise devices and methods, objects of this invention are to provide a chest exerciser which: Is a pneumatic resister of linear motion; Provides selectively push exercise and pull exercise; Provides selective levels of push and pull resistance; Is low-weight, small and inexpensive; Has selective handle means that are positional on opposite ends for different individual characteristics and for application to different muscles of the chest area; and Has selective attachment and/or anchor means that are positional on opposite ends for different individual characteristics and for application to select muscle groups and to specific muscles of the select muscle groups in a body. [0019] This invention accomplishes these and other objectives with a chest exerciser having a pneumatic resister of linear motion and a selection of handle, attachment and/or anchor means that are positional on opposite ends for direction of selectively push exercise and pull exercise to select muscle groups and to specific muscles in the select muscle groups. The pneumatic resister of linear motion can be a cylindrical pump, a bellows pump, a variously telescopic pump, a resilient pump or other pneumatic pump or combinations of pneumatic pumps. The pneumatic resister of linear motion can be selectively resistant to linear motion by means of flow resisters that can be adjustable of resistance or rate of inflow and outflow of air. Separate inflow and outflow resisters can be provided for adjustment of push and pull exercise. Handle means can be different for different sizes of hands and for different Mathéo Software (c) http://www.matheo-software.com 35/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) positioning of the pneumatic resister in relation to muscles. Attachment and anchor means can be provided for positioning the pneumatic resister in desired relationship to select muscle groups and to specific muscles in muscle groups of a body. [0020] The invention provides an exercise device comprising: an elongated plunger housing having a first end and second end; a first hand grip member and a first plunger, the first hand grip member connected to the first plunger; and a second hand grip member and an air volume closure member, the second hand grip member connected to the air volume closure member; the first plunger in sealed sliding contact with the plunger housing; a common air volume enclosed by the plunger housing, the first plunger, and the common air volume closure member; the exerciser sized and shaped to be handheld by an individual for exercising. Preferably, the exercise device further comprises an adjustable vent for venting the plunger housing. Preferably, the air volume closure member is attached to the second end of the elongated plunger housing. Preferably, the adjustable vent comprises a first adjustable vent formed in the air volume closure member. Preferably, the exercise devise further includes a plunger housing first end wall member, the first plunger is connected to the hand grip member via a plunger connector passing through the plunger housing first end wall member, wherein the air volume closure member forms a plunger housing second end wall, and the adjustable vent further includes a second adjustable vent in the plunger housing first end wall member. Preferably, the air volume closure member comprises a second plunger in sealed sliding contact with the plunger housing. Preferably, the second plunger is located on the opposite side of the common air volume from the first plunger. [0021] Preferably, the exercise device includes an elongated plunger housing having a first end and a second end; a first plunger and a first plunger hand grip member, the first plunger hand grip member attached to the first plunger; a second plunger and a second plunger hand grip member, the second plunger hand grip member connected to the second, plunger; and the first plunger is in sealed sliding contact with the first end of the plunger housing and the second plunger in sealed sliding contact with the second end of the plunger housing with the first and second plungers opposed on opposite sides of a common air volume within the plunger housing; the exerciser sized and shaped to be hand-held by an individual for exercising. [0022] Preferably the exercise device further includes an adjustable vent for venting the plunger housing. Also preferably, the adjustable vent includes a first adjustable vent for venting the first end of the plunger housing and a second adjustable vent for venting the second end of the plunger housing. Preferably, the adjustable vent communicates with the common air volume. Preferably, the adjustable vent is located centrally to the elongated plunger housing. Preferably, the adjustable vent includes a vent member attached to a knob and a channel communicating with the common air volume, the vent member having a plurality of holes of different sizes, the channel and vent member arranged so that as the knob is turned, a different one of the holes aligns with the channel. Preferably, the first plunger hand grip member and the second plunger hand grip member move relative to each other between a fully extended position and a fully retracted position, and wherein in the fully retracted position the retracted grip distance between the proximal end of the first plunger hand grip member and the proximal end of the second plunger hand grip member is between 0 and 10 inches. Preferably, the retracted grip distance is between 1 and 5 inches. Preferably, the elongated plunger housing comprises two housing members joined by a seal. Preferably, the elongated plunger housing include a vent hole in the elongated plunger housing. Also preferably, the plunger hand grip members each include a plunger hand grip member vent hole. [0023] The invention also provides a method for enhancing the size of a breast comprising: activating a breast growth enhancement compound in the breast of a user; holding with one hand a first hand grip member attached to a first plunger; holding with the other hand a second hand grip member attached to a second plunger, the first plunger opposite the second plunger and in sealed sliding contact in an elongated plunger housing having an adjustable vent for venting the air in the elongated plunger housing; and moving the first plunger and second plunger to move in reciprocating motions relative to each other. Preferably, the activating comprises a process selected from the group consisting of: ingesting herbal foods containing the compound, ingesting pills containing the compound, applying a crème containing the compound to the breast, applying a tonic containing the compound to the breast, and applying a paste containing the compound to the breast. [0024] The invention further provides a method for using a breast enhancement system having: a first hand-held reciprocating member comprising a first proximal end and a first distal end; a second hand-held reciprocating member comprising a second proximal end and a second distal end, wherein first hand-held reciprocating member and the second hand-held reciprocating member being in a fully retracted position, the first proximal end and second proximal end define a retracted gap distance of 0 to 10 inches; and a two-way resistive force member for generating a force that opposes reciprocating Mathéo Software (c) http://www.matheo-software.com 36/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) movement of the first hand-held reciprocating member and the second hand-held reciprocating member, in response to the reciprocating movement of the first hand-held reciprocating member and the second hand-held reciprocating member; the method comprising: activating a breast growth enhancement compound in the breast of a user; grasping with one hand the first hand-held reciprocating member; grasping with the other hand second hand-held reciprocating member; and moving the first hand-held reciprocating member and second hand-held reciprocating member in reciprocating motion relative to each other. Preferably, the retracted grip distance is between 1 and 5 inches. Preferably, the activating comprises a process selected from the group consisting of: ingesting herbal foods containing the compound, ingesting pills containing the compound, applying a crème containing the compound to the breast, applying a tonic containing the compound to the breast, and applying a paste containing the compound to the breast. Preferably, the compound comprises pueraria mirifica. [0025] In another aspect the invention provides a method for enhancing the size of breasts comprising: applying a breast growth enhancement topical cream to the breasts of a user; holding with one hand a first end of a reciprocating two-ended exercise device; holding with the other hand a second end of the reciprocating two-ended exercise device; stimulating breast enhancement by exerting reciprocating motion by the one hand and the other hand on the reciprocating two-ended exercise device, the two-ended exercise device capable of enabling the one hand and the other hand to be in sufficiently close proximity with each other during a compression stroke of the reciprocating motion to focus exercise on the breast area of the user. Preferably, the topical cream comprises pueraria mirifica. [0026] The above and other objects, features and advantages of the present invention should become even more readily apparent to those skilled in the art upon a reading of the following detailed description in conjunction with the drawings wherein there is shown and described illustrative embodiments of the invention. BRIEF DESCRIPTION OF THE DRAWINGS [0027] This invention is described by appended claims in relation to description of a preferred embodiment with reference to the following drawings which are described briefly as follows: [0028] FIG. 1 is a side view of an embodiment with an accordion pneumatic resister of linear motion; [0029] FIG. 2 is a partially cutaway side view of an embodiment with a telescopic pneumatic resister of linear motion; [0030] FIG. 3 is a partially cutaway side view of an embodiment with a single-plunger-pump pneumatic resister of linear motion; [0031] FIG. 4 is a partially cutaway side view of an embodiment with a double-plunger-pump pneumatic resister of linear motion; [0032] FIG. 5 is an exploded fragmentary sectional view of an inflow resister and an outflow resister in relation to an internal periphery of a pneumatic resister of linear motion; [0033] FIG. 6 is a fragmentary top view of an inflow resistor; [0034] FIG. 7 is a fragmentary top view of an outflow resister; [0035] FIG. 8 is a partially cutaway elevation view of a pneumatic resister of linear motion with quick-disconnect attachments for handles and other exercise components; [0036] FIG. 9 is a top view of an alternative quick-disconnect attachment; [0037] FIG. 10 is an elevation view of a stick representation of an exerciser using this invention for separate chest exercises; [0038] FIG. 11 is an elevation view of a stick representation of an exerciser using this invention for lifting and pressing exercises in a standing position; [0039] FIG. 12 is an elevation view of a stick representation of an exerciser using this invention for lifting and pressing exercises in a prone position; [0040] FIG. 13 is an elevation view of a stick representation of an exerciser using this invention for lifting and pressing exercises between feet and shoulders in a sitting position; [0041] FIG. 14 is an elevation view of a stick representation of an exerciser using this invention for lifting and pressing exercises between knees and shoulders in a sitting position; [0042] FIG. 15 is a side view of a dual cylinder pneumatic resister exercise device in the fully closed position; [0043] FIG. 16 is a side cut-away view showing a single action double plunger embodiment of the pneumatic resister exerciser device; [0044] FIG. 17 is a side cut-away view of a double-action, double plunger embodiment of the pneumatic resister exercise device; Mathéo Software (c) http://www.matheo-software.com 37/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) [0045] FIG. 18 is a side cut-away view of a spring assisted single-action, double-plunger embodiment of the pneumatic resister exerciser device; [0046] FIG. 19 is a partially cut-away side view of an embodiment with a double-plunger-pump pneumatic resister of linear motion with closely retracted handle locations; [0047] FIG. 20 is front view of a double-plunger embodiment of the pneumatic resister exerciser device with closely retracted handle locations and adjustable vent; [0048] FIG. 21 is a side cut-away view of a double-plunger embodiment of the pneumatic resister exerciser device with closely retracted handle locations and adjustable vent; [0049] FIG. 22 is a front view of a double-plunger embodiment of the pneumatic resister exerciser device with extended handle locations and adjustable vent; [0050] FIG. 23 is a side cut-away view of a double-plunger embodiment of the pneumatic resister device with extended handle locations and adjustable vent; [0051] FIG. 24 is an end view of a double-plunger embodiment of the pneumatic resister device with adjustable vent; [0052] FIG. 25 is an exploded view of a double-plunger embodiment of the pneumatic resister and adjustable vent; [0053] FIG. 26 is a front view of the vent member depicting a plurality of holes therein; and [0054] FIG. 27 is a perspective end view of plunger hand grip member depicting vent holes therein. DESCRIPTION OF PREFERRED EMBODIMENT [0055] Reference is made first to FIGS. 1-4. A pneumatic resister 1 of linear motion is a select type of pneumatic pressurizer that is operated linearly with pneumatic resistance of airflow through a design form of flow regulator with at least one inflow resister 2 and/or at least one outflow resister 3. Examples of select pneumatic resisters 1 are an accordion pneumatic resister 4 shown in FIG. 1, a telescopic pneumatic resister 5 shown in FIG. 2, a single-plunger pneumatic resister 6 shown in FIG. 3, and a double-plunger pneumatic resister 7 shown in FIG. 4. In a preferred embodiment as a chest exerciser, all pneumatic resisters 1 depicted in FIGS. 1-4 have a first hand grip member 8 and a second hand grip member 9 that are attached to end plates 10 or to plunger shafts 11, respectively, for separate types of pneumatic resisters 1 as illustrated. End walls 10 are preferred design walls of different types of pneumatic resisters for positioning of inflow resisters 2 and outflow resisters 3. Each of the embodiments of FIGS. 3 and 4 included an elongated plunger housing 6 and 7, respectively. In the embodiment of FIG. 3 a first plunger 11A is in sealed sliding contact with plunger housing 6, and in the embodiment of FIG. 4, a first plunger 11B and a second plunger 11C are in sealed sliding contact with plunger housing 7. In the embodiment of FIG. 3 a common air volume 6A, is enclosed by plunger housing 6, first plunger 11A, and common air volume closure member 10, which in this embodiment is the plunger housing end wall. In the embodiment of FIG. 4, a common air volume 7A, is enclosed by plunger housing 7, first plunger 11B, and common air volume closure member 11C, which in this embodiment is second plunger 11C. [0056] Referring to FIGS. 5-7, a selection of flow-resistant means such as inflow resister 2 and outflow resister 3 are foreseeable. An inflow resister 2 can be a type that has an inlet valve 12 which seats against an inlet valve seat 13 in a threaded inlet-adjustment sleeve 14 having an adjustment knob 15 that is preferably knurled. An inlet-valve spring 16 can be anchored in an inlet-orifice step 17. Inward threading of the inlet-adjustment sleeve 14 increases spring pressure against the inlet valve 12 to increase suction pressure required for volume expansion of whichever pneumatic resister 1 is employed for increasing pull resistance. The converse is employed for decreasing pull resistance. A sufficiently rigid inlet-valve spring 16 to function as a rigid sleeve converts inflow resistance from spring-operational mode to volume-operational mode because the inlet valve 12 then would be held rigidly at an adjusted distance from the inlet-valve seat 13. Spring-operational mode with a designedly lighter inlet-valve spring 16 is preferable because it is near totally pressure sensitive instead of near totally speed sensitive in comparison to volume-operational mode. The inlet-valve seat 13 surrounds an inlet-valve orifice 18 through which inflow air is directed to inlet orifice 19 in an end plate 10. [0057] An outflow resister 3 can be a type that has an outlet valve 20 which seats against an outletvalve seat 21 surrounding an outlet orifice 22 in the end plate 10. A threaded outlet-adjustment sleeve 23 has an outlet-valve orifice 24 surrounded by an outlet-spring step 25 against which an outlet spring 26 is anchored to apply opening pressure against the outlet valve 20 in accordance with threaded positioning of the outlet-adjustment sleeve 23. An adjustment knob 15 can be the same for both the inflow resister 2 and the outflow resister 3. Also like the inflow resister 2, volume-operational mode is achievable with a sufficiently rigid outlet spring 26 for the same reasons in reverse flow. [0058] Referring to FIGS. 6-7, rotational indicia 27 and a pointer 28 on adjustment knobs 15 can be provided for fine-tune adjustment. Extra-fine threading of the inlet-adjustment sleeve 14 and the outlet- Mathéo Software (c) http://www.matheo-software.com 38/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) adjustment sleeve 23 is recommended for two reasons. First, it provides a lower cam angle for high positioning reliability. Second, it provides finer adjustment which is important because minute differences in valve openings make a big difference in airflow that affects pneumatic resistance. From the top, a uniformity of appearance of valves that are easy to set enhances product acceptance and marketing. [0059] Referring to FIGS. 8-9, handles 8 and 9 can be attached to end plates 10 and to plunger shafts 11 with an attachment means, such as a quick-disconnect attachment 29, threaded attachments or other means. Other types of quick disconnect devices than the one depicted also can be employed. For example, a Luer connector that is used in the medical profession for syringes is good because it is particularly rigid. A plausible modification of a Luer connector would be a tapering of a connector shaft 30 and a matched tapering of a cylindrical receptor 31 that are shown straight as employed generally in mechanical fields. The tapering would be at distal ends of the connector shafts, 30 and at proximal ends of the cylindrical receptors 31 beyond a lock pin 32 and a lock slot 33. [0060] An attachment means such as a form of quick-disconnect attachment 29 allows use of bodyconnection components and body anchors such as body anchor 34 and multiple handles 8 and 9 on either end. Other body-connection components also can be attached to either or both ends of a pneumatic resister 1 with an attachment means that is standard for different handle and bodyconnection components. Foot straps 35 can be modified to be leg straps or shoulder attachments as optional types of body-connection components. Shoulder attachments, for instance, would permit shoulder and chest exercise independently of arm exercise when desired for chest buildup. Standardized attachment means opens this invention up to an equivalent of weight-lifting and pressing exercise simultaneously with adjustable resistance in opposite directions. This is a feat never before accomplished with such a convenient and highly versatile exerciser. [0061] Reference is made now to FIGS. 10-14 which depict stick figures of exercisers in relation to methods for using a pneumatic resister 1 having a first attachment 36 and a second attachment 37 that are either handles 8 and 9 or body anchors 34 as appropriate for particular methods of use. In FIG. 10, a method for exercising chest and arm muscles is outlined by arms 38 of an exerciser 39 grasping first attachments 36 and second attachments 37, which in this method would be handles 8 and 9, and then pushing and pulling the attachments 36 and 37 at arm heights which cause exercise of different parts of the exerciser's chest area. Different positions are shown in dashed lines. [0062] In FIG. 11, a foot anchor 40 is attached to a second attachment 37 while a first attachment 36, preferably two handles 8 and 9 attached to a body anchor 34 as depicted in FIG. 8, is grasped for lifting and pressing exercise in a standing or variously upright position of the exerciser 39. [0063] In FIG. 12, similar methodical relationships are depicted as for FIG. 11, but with the exerciser 39 on a platform 41 such as an exercise pad or a bed to achieve different exercise effects for different types of people with different exercise objectives. [0064] In FIG. 13, the exerciser 39 is sitting for selectively different exercising with components similar to those explained in relation to FIGS. 11-12. [0065] In FIG. 14, the exerciser 39 is sitting with a leg anchor 42 attached to the second attachment 37. Although shown with arms 38 in contact with the first attachment 36, the method shown here is particularly appropriate for a body anchor 34 shown in FIG. 8 for connection to shoulders of an exerciser 39 for chest exercise that is independent of arm and leg work. [0066] In FIG. 15 a side view of the exterior of a single-action, double-plunger embodiment is shown having a fixed handle 102 with hand grip 104 on one end and a moveable handle 103 with hand grip 105 on the other end. The body of the device 101 has an upper cylinder 106 and a lower cylinder 107 connected by central section 108. A resister adjustment valve 109 in the central section 108 may be turned to adjust air flow through the valve, which in turn increases or reduces the pneumatic resistance when the moveable handle 103 is pulled outward from the body of the device 101. In other words, the more air that is vented out the valve 109 the less resistance there will be against the push or pull of a user's muscles. The valve 109 may be a standard petcock valve and have multiple positions such as high, medium or low depending on the resistance desired. [0067] In FIG. 16 the internal workings of the single-action, double-plunger embodiment shown in FIG. 15 are shown. The upper cylinder 106 and lower cylinder 107 contain rods 111a and 111b with plungers 110a and 110b mounted on the ends thereof. Air vents 112 on each end adjacent to each handle are the central connecting air vent 113 allow compressed air to pass to the vent 109 for resistance adjustment purposes. In operation when the moveable handle 103 is pulled away from the body 101 the air behind the plunger is adjustably compressed to provide inward resistance against the muscles. Conversely, when the removable handle 103 is pushed inward from an extended outward position the air in front of the plungers 110a and 110b is also compressed and forced through the vent 109 to provide adjustable resistance. Mathéo Software (c) http://www.matheo-software.com 39/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) [0068] In FIG. 17 a double-action version of the pneumatic resister exercise device of the present invention is illustrated wherein both handles 102 and 103 may be simultaneously pulled outward away from the body 101 or conversely pushed inward simultaneously by wrapping the fingers around the grips 104 and 105. As illustrated, this version has top and bottom cylinders of the body split into four pneumatic sections, 121a and 121b on the bottom and 121c and 121d on the top, supported by central walls 116a and 116b. Each cylinder has a plunger 114a, 114b, 114c and 114d mounted on rods 115a, 115b, 115c and 115d. Central air vents 117a and end air vents 118 connected to central vent 113 are provided to allow air to be vented through central valve 109 to adjustably control the resistance of the device. In operation when the handles 102 and 103 are pulled outward away from the body 101, air behind the plungers 114a, 114b, 114c and 114d is compressed with air being vented out of the valve 109 through vents 118 and 113 depending on the adjustable setting of the valve 109. Conversely, when the handles 102 and 103 are pushed inward from an outward extension the air in front of the plungers 114a, 114b, 114c and 114d is compressed against the central walls 116a and 116b and vented out the valve 109 through central plunger vents 117a and 117b. Thus, in the latter manner this pneumaticexercise devices provides resistance in both directions, inward and outward, to exercise different muscles of the body, particularly the chest. [0069] In FIG. 18 the single-action embodiment of the present invention previously illustrated in FIGS. 15 and 16 are shown being assisted by springs 119a and 119b and 120a and 120b. Springs 120a and 120b behind the plungers 110a and 110badd resistance force when the handle 103 is being pulled outward from the body 101 of the device. Conversely, this resistance may be somewhat offset by the outward force of the springs 119a and 119b in front of the plungers 110a and 110b. Conversely, when the handles 103 are pushed inward toward the body 101 from an outward extending position, resistance is provided by the springs 119a and 119b in front of the plungers 110a and 110b. Although FIG. 18 shows springs both in front of and behind the plungers, either or any combination may be used with or without the central valve 109, which also acts to increase or decrease resistance. [0070] Although. FIGS. 15, 16, 17 and 18 showing single or double action using two or fourcylinders, a plurality of cylinders with plungers could be utilized to achieve the pneumatic resistance provided by this device. In any event, these double plunger versions provide more stability for the exerciser not provided by a single plunger version described and illustrated in FIGS. 1-4 as the inward or outer pressure against the handles does not result in the transverse movement which could cause the pneumatic exercise device to fall from one's hands during use. Another embodiment of the pneumatic exercise device that is designed with handles that can be closely retracted and provide stability while exercising the breast muscles and enhancing the breast size of a user is described below. [0071] In FIG. 19, a partially cutaway side view of another embodiment of the pneumatic exercise device is shown. Double-plunger-pump pneumatic resister 200 includes a first plunger hand grip member 206 and a second plunger hand grip member 204 wherein a cross-section of the members 206 and 204 form a circle, or alternatively, other geometric shapes such as ovals, pentagons, hexagons, hectagons, octagons or squares. A first handle 224 and a second handle 226 provide a grasping surface for the first plunger hand grip member 206 and the second plunger hand grip member 206, respectively. The first handle 224 and second handle 226 are made from a foam material, or alternatively, other compressible or non-compressible material such as plastic, Styrofoam, rubber, sponge, foam rubber or sponge rubber. [0072] The double-plunger-pump pneumatic resister 200 also includes a plunger housing 228 that contains a first plunger 211 and a second plunger 213. The first plunger 211 is attached to the first plunger hand grip member 206 by plunger connector 210 at the distal end of the first plunger assembly. Also, the second plunger 213 is attached to the second plunger hand grip member 204 at the distal end of the second plunger 212. Both the first plunger hand grip member 206 and the second hand grip member 204 include holes 230 to allow air flow. The plunger housing 228 is enclosed at one end by a first end wall 208 and at the other end by a second end wall 222. Both first end wall 208 and second end wall 222 have holes to allow for the first plunger 212 and second plunger 210, respectively, to move through. Preferably, the first end wall 208 includes a first adjustable inlet vent 218 and a first adjustable outlet vent 220, and the second end wall 222 includes a second adjustable inlet vent 214 and a second adjustable outlet vent 216 to allow the user to set the, desired airflow resistance of the pneumatic exerciser device. In this embodiment, a common air volume 232, is enclosed by plunger housing 228, first plunger 211, and common air volume closure member 213, which in this embodiment is second plunger 213. This embodiment of the pneumatic exercise device is designed with handles that can be closely retracted and provide stability while exercising the breast muscles and enhancing the breast size of a user. When the pneumatic exerciser device is in a fully retracted position, there is a retracted grip distance between the proximal end 240 of the first plunger hand grip member 224 and the proximal end 241 of the second plunger hand grip member 226. This retracted grip Mathéo Software (c) http://www.matheo-software.com 40/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) distance is preferably between 0 and 10 inches. Preferably, the retracted grip distance is between 1 and 5 inches. [0073] FIG. 20 is a front view of another embodiment of the pneumatic, exerciser device in a fully retracted position. The double-plunger-pump pneumatic resister 300 includes a first plunger hand grip member 302 and a second plunger hand grip member 304. The first plunger hand grip member 302 includes a first handle 306 and a first end cap 314 and the second plunger hand grip member 304 includes a second handle 308 and a second end cap 316. When the pneumatic exerciser device is in a fully retracted position, there is a retracted grip distance 311 between the proximal end of the first plunger hand grip member 302 and the proximal end of the second plunger hand grip member 304. This retracted grip distance 311 is preferably between. 0 and 10 inches. Preferably, the retracted grip distance 311 is between 1 and 5 inches. [0074] The double-plunger-pump pneumatic resister 300 further includes an elongated plunger housing 310 including a flared vent housing 319 which encloses an adjustable vent 312. A side cutaway view of double-plunger-pump pneumatic resister 300 is shown in FIG. 21. In addition to the parts described above in FIG. 20, FIG. 21 depicts a first plunger 312 and a second plunger 314 in sealed sliding contact with the elongated plunger housing 310. Here "sealed" sliding contact means that, as it slides, the plunger is in suitable contact with the housing so that air or other fluid cannot circumvent the plunger by escape where the plunger contacts the housing, but rather is pushed by the plunger as it moves. Of, course, in the real physical world, every plunger is to some extent imperfect, and thus this, term includes plungers in which there may be an insignificant amount of air or other gas that leaks through the plunger seal. Also shown is an optional recessed area 307, perhaps best shown in FIG. 25, in the first plunger hand grip member 302 and the second hand grip member 304 to allow the first handle 306 and the second handle 308 to be flush with the first plunger hand grip member 302 and the second plunger hand grip member 304, respectively. The elongated plunger housing 310 may be a onepiece assembly or a multi-piece assembly. When the elongated plunger housing 310 is a two-piece assembly, as shown in FIGS. 21 and 25, a seal 322 is placed between the proximal ends, such as 321 (FIG. 25) to seal the elongated plunger housing 310. The seal 322 includes a channel 324 to communicate airflow between the seal and the adjustable vent 312. The first plunger hand grip member 302 and the second plunger hand grip member 304 move between fully retracted positions and fully extended positions. [0075] In FIG. 22, a front view is shown of the double-plunger-pump pneumatic resister 300 in a fully extended position revealing a first end 310A and a second end 310B of the two-piece elongated plunger housing 310. The first plunger hand grip member 302 and the second plunger hand grip member 304 are shown extended distally relative to the center of the elongated plunger housing 310. In FIG. 23, the common air volume 326 is shown between the first plunger 330 and the common air volume closure member 331, which in this embodiment is the second plunger 331. The common air volume 326 increases in size as the first plunger 330 and second plunger 321 are moved away from each other and, conversely, decreases in size as the first plunger 330 and second plunger 331 are moved toward each other. As the user moves the first plunger hand grip member 302 and the second plunger hand grip member 304 away from each other, air is introduced into the common air volume 326 via the adjustable vent 312 creating a resistive motion for the user to overcome. Conversely, as the user moves the first plunger hand grip member 302 and the second plunger hand grip member 304 toward each other, air is evacuated from the common air volume 326, via the adjustable vent 312 creating a resistive motion for the user to overcome. In FIG. 24, the double-plunger-pump pneumatic resister 300 is shown from an end view revealing a front view of the first end cap 314. The double-plunger-pump pneumatic resister 300 includes several internal parts that are interrelated to the resistive force and are described below. [0076] In FIG. 25, an exploded view of the double-plunger-pump pneumatic resister 300 is shown. A two-piece elongated plunger housing 310 is shown as separated in the middle, depicting the first housing member 310A and the second housing member 310B which together comprise the two-piece elongated plunger housing 310. Both the first housing member 310A and the second housing member 310B include at least one vent hole 317. (FIG. 24A) located at the distal end of the respective housing member to vent pressure created between the plunger, such as 330, and the distal end 325 of the respective housing member. The first housing member 310A, second housing member 310B, the first plunger hand grip member 302 and the second plunger hand grip member 304 can be made of plastic, or alternatively, by other rigid material such as steel, aluminum, metal, carbon, plexiglass or fiberglass. The first housing member 310A and the second housing member 310B are connected by a seal 322 that seals the two housing members creating the common air volume 326. In an alternative embodiment this seal can be a divider which divides the air volume into separate volumes 354 and 355. Mathéo Software (c) http://www.matheo-software.com 41/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) [0077] Seal 322 preferably includes an O-ring groove 340. The O-ring groove 340 can be on one or both sides of the seal 322 and accepts an O-ring for sealing the first housing member 310A and second housing member 310B when they are positioned together to form the elongated plunger housing 310. The first housing member 310A contains the first plunger 331 in sealed slideable contact. The first plunger assembly includes a first plunger connector shaft 328, a first plunger 330 and a first plunger end O-ring 332. The second housing member 310B contains the second plunger 331 in sealed slideable contact. The second plunger assembly includes the same structure and parts as the first plunger assembly and will not be described. The adjustable vent 312 includes a knob 334 that enables the user to adjust the pneumatic resistance of the double-plunger-pump pneumatic resister 300. The knob 334 allows the user to increase or decrease the resistive force of the double-plunger-pump pneumatic resister 300. [0078] The adjustable vent 312 includes a vent member 336 attached to a knob 334. The vent member 336 and knob 334 are attached to each other and rotate together to adjust airflow resistance of the double-plunger-pump pneumatic resister 300. The knob 334 is in communication with the seal 322 via channel 324. In FIG. 26, a front view of the vent member 336 is shown depicting a plurality of varying sized vent member holes 342. The vent member holes 342 are located around the perimeter of the vent member 336. The channel 324 is tangentially offset from the middle of the seal to conform to the location of at least one of the vent member holes 342. As the knob 334 is rotated or selected by the user, a corresponding vent member hole, 342 is selected, which comes in communication with the channel 324, thereby creating greater or lesser pneumatic resistive force through the adjustable vent 312. In. FIG. 27, a perspective view of the second plunger hand grip member 304 is shown with the second end cap 316 removed to expose at least one second plunger hand grip member vent hole 344. As mentioned above, the first plunger hand grip member 302 includes at least one first plunger hand grip member vent hole and the second plunger hand grip member 304 includes at least one second plunger hand grip member vent hole. The first end cap 314 and second end cap 316 are supported from their respective plunger hand grip members by ribs 315 (FIG. 25), or alternatively, other such devices to allow for airflow between the end caps 314 and 316 and their respective plunger hand grip members 302 and 304. At the distal end, such as 325, or each plunger housing, such as 310, the end cap 314 is supported by the plunger hand grip member, such as 304, and the plunger connector shaft, such as 328, to create an air passage way for venting the plunger hand grip member vent hole 344 and the at least one housing vent hole 317. The system 300 is held together by screws that screw into bosses, such as 323, within flared vent housing 319. [0079] The present invention includes a system for enhancing the size of a breast of the user. The system includes the use of an exerciser as described herein in combination with a breast nutrient compound, such as pueraria mirifica, a known natural plant estrogen. [0080] As is known in the art, some nutrient compounds, such as pueraria mirifica, can enhance breast growth of a user. Pueraria mirifica is a natural plant estrogen, also known as phytoestrogens, which are constituents that bind to estrogen receptors and have other effects similar to estrogen in the body. They are not truly estrogens, since their chemical forms are different from estrogen. These phytoestrogens do have estrogen-like effects that can be very helpful for the growth of breast cells. Estrogen is a natural body substance that affects the human body characteristics, including growth and maintenance of all female sex characteristics. The deficiency of estrogen in the body can cause menopause, sagging breasts, wrinkled skin and bone loss. [0081] Pueraria mirifica can lengthen the milk ducts of a breast and stimulate and expand the fat tissues, resulting in firmer breasts. Pueraria mirifica can also maintain collagen, develop new skin cells, which can both contribute to soft, smooth and beautiful breasts. The active ingredients in these pueraria mirificas include miroestrol, pueraria, mirifica, daidazein, B-sitistirol, soumestrol, genistein and genisten. The pueraria mirifica can be delivered to the breast area via various mechanisms including ingesting herbal foods, ingesting pills, application of crèmes, application of tonics and application of pastes. [0082] The pueraria mirifica can be applied directly onto the breast of a user via a crème or tonic. Also, the pueraria mirifica can be taken orally, including by herbal foods and pills. The pueraria mirifica promotes fibroblasts in normal breast cells. The pueraria mirifica stimulates fibroblasts that produce collagen tissues or glands. Working together, the pueraria mirifica and the pneumatic exerciser embody an effective system that can increase the breast size of a user. This system offers the user a plethora of methods in which to enhance the size of the user's breast. One such embodiment is to apply pueraria mirifica crème directly to the breast of a user followed by a regimen of alternating extending and retracting forces applied to the pneumatic exerciser by the user. For stronger users, the knob 334 is selected to a more resistive force, and conversely for less strong users, the knob 334 is selected to a less Mathéo Software (c) http://www.matheo-software.com 42/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) resistive force. This method of using the pueraria mirifica and pneumatic exerciser system can be repeated several times a day for improved results. [0083] Another embodiment method for enhancing the breast size of a user involves ingesting a pill containing the pueraria mirifica followed by the above stated regimen of alternating extending and retracting forces applied to the pneumatic exerciser by the user. In addition, a further embodiment involves digesting herbal foods that contain pueraria mirifica followed by the above stated regimen of alternating extending and retracting forces applied to the pneumatic exerciser by the user. [0084] A feature of the invention is the close proximity of the handles, such as 8, 9, 224, 226, 306, 308, particularly in the embodiments of FIGS. 4, and 19-27, which focuses the exercise at the breast area of the user. This proximity is enhanced by the fact that the exerciser utilizes a common air volume, thereby eliminating parts that force the handles to be further apart. This is also enhanced by the fact the handles, such as 224, 226, 306, 308 slide on and are coaxial with the plunger housing, such as 228, 310A and 310B. This focus of the exerciser on the breast area in combination with the use of a breast nutrient compound provides a synergistic effect that is not available with any prior art breast enhancement system. [0085] A new and useful chest exerciser having been described, all such modifications, adaptations, substitutions of equivalents, combinations of parts, pluralities of parts, applications and forms thereof as described by the following claims are included in this invention.Data supplied from the esp@cenet database - Worldwide Claims: 1-22. (canceled) 23. A method for enhancing the size of a breast comprising: activating a breast growth enhancement compound in the breast of a user, holding with one hand a first hand grip member attached to a first plunger, holding with the other hand a second hand grip member attached to a second plunger, said first plunger opposite said second plunger and in sealed sliding contact in an elongated plunger housing having an adjustable vent for venting the air in said elongated plunger housing; and moving said first plunger and second plunger to move in reciprocating motions relative to each other. 24. A method as in claim 23 wherein said activating comprises a process selected from the group consisting of: ingesting herbal foods containing said compound, ingesting pills containing said compound, applying a crème containing said compound to said breast, applying a tonic containing said compound to said breast, and applying a paste containing said compound to said breast. 25. A method for using a breast enhancement system having: an elongated plunger housing having a first end and second end; a first hand grip member and a first plunger, said first hand grip member connected to said first plunger, a second hand grip member and an air volume closure member, said second hand grip member connected to said air volume closure member, said first plunger in sealed sliding contact with said plunger housing; a common air volume enclosed by said plunger housing, said first plunger, and said common air volume closure member, said exerciser sized and shaped to be hand-held by an individual for exercising; and a breast growth enhancement compound; the method comprising: activating said breast growth enhancement compound in the breast of a user, grasping with one hand said first hand grip member, grasping with the other hand said second hand grip member, and moving said first hand grip member and second hand grip member in reciprocating motion relative to each other. 26. A method as in claim 25 wherein said activating comprises a process selected from the group consisting of: ingesting herbal foods containing said compound, ingesting pills containing said compound, applying a crème containing said compound to said breast, applying a tonic containing said compound to said breast, and applying a paste containing said compound to said breast. 27. A method for using a breast enhancement system having: a first hand-held reciprocating member comprising a first proximal end and a first distal end; Mathéo Software (c) http://www.matheo-software.com 43/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) a second hand-held reciprocating member comprising a second proximal end and a second distal end, wherein first hand-held reciprocating member and said second hand-held reciprocating member being in a fully retracted position, the first proximal end and second proximal end define a retracted gap distance of 0 to 10 inches; and a two-way resistive force member for generating a force that opposes reciprocating movement of said first hand-held reciprocating member and said second hand-held reciprocating member, in response to the reciprocating movement of said first hand-held reciprocating member and said second hand-held reciprocating member, the method comprising: activating a breast growth enhancement compound in the breast of a user, grasping with one hand said first hand-held reciprocating member, grasping with the other hand second hand-held reciprocating member, and moving said first hand-held reciprocating member and second hand-held reciprocating member in reciprocating motion relative to each other. 28. A method as in claim 27 wherein said retracted grip distance is between 1 and 5 inches. 29. A method as in claim 27 wherein said activating comprises a process selected from the group consisting of: ingesting herbal foods containing said compound, ingesting pills containing said compound, applying a crème containing said compound to said breast, applying a tonic containing said compound to said breast, and applying a paste containing said compound to said breast. 30. A method as in claim 29 wherein said compound comprises pueraria mirifica. 31. A method for enhancing the size of breasts comprising: applying a breast growth enhancement topical cream to the breasts of a user, holding with one hand a first end of a reciprocating two-ended exercise device; holding with the other hand a second end of said reciprocating two-ended exercise device; stimulating breast enhancement by exerting reciprocating motion by said one hand and said other hand on said reciprocating two-ended exercise device, said two-ended exercise device capable of enabling said one hand and said other hand to be in sufficiently close proximity with each other during a compression stroke of said reciprocating motion to focus exercise on the breast area of the user. 32. A method as in claim 31 wherein said topical cream comprises pueraria mirifica. 33. A method for using a breast enhancement system having: an elongated plunger housing having a first end and second end; a first plunger and a first plunger hand grip, said first plunger hand grip member connected to said first plunger, and a second plunger and a second plunger hand grip member, said second hand grip member connected to said second plunger, said first plunger in sealed sliding contact with said first end of said plunger housing and said second plunger in sealed sliding contact with said second end of said plunger housing with said first and second plungers opposed on opposite sides of an air volume with said plunger housing; and a breast growth enhancement compound; the method comprising: activating said breast growth enhancement compound in the breast of a user, grasping with one hand said first hand grip member, grasping with the other hand said second hand grip member, and moving said first hand grip member and second hand grip member in reciprocating motion relative to each other. 34. A method as in claim 33 wherein said activating comprises a process selected from the group consisting of: ingesting herbal foods containing said compound, ingesting pills containing said compound, applying a crème containing said compound to said breast, applying a tonic containing said compound to said breast, and applying a paste containing said compound to said breast. 35. A method as in claim 33 wherein said compound comprises pueraria mirifica. 36. A method for using a breast enhancement system having: an elongated plunger housing having a first end and second end; Mathéo Software (c) http://www.matheo-software.com 44/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) a first plunger and a first plunger hand grip, said first plunger hand grip member connected to said first plunger, a second plunger and a second plunger hand grip member, said second hand grip member connected to said second plunger, said first plunger in sealed sliding contact with said first end of said plunger housing and said second plunger in sealed sliding contact with said second end of said plunger housing with said first and second plungers opposed on opposite sides of an air volume with said plunger housing; said exerciser sized and shaped to be hand-held by an individual for exercising; and a breast growth enhancement compound; the method comprising: activating said breast growth enhancement compound in the breast of a user, grasping with one hand said first hand grip member, grasping with the other hand said second hand grip member, and moving said first hand grip member and second hand grip member in reciprocating motion relative to each other. 37. A method as in claim 36 wherein said activating comprises a process selected from the group consisting of: ingesting herbal foods containing said compound, ingesting pills containing said compound, applying a crème containing said compound to said breast, applying a tonic containing said compound to said breast, and applying a paste containing said compound to said breast.Data supplied from the esp@cenet database - Worldwide Mathéo Software (c) http://www.matheo-software.com 45/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 16. WO2005084340 - 9/15/2005 PUERARIA CANDOLLEI VAR. MIRIFICA A SHAW. & SUVAT. EXTRACT URL EPO = http://v3.espacenet.com/textdoc?F=3&CY=ep&LG=en&IDX=WO2005084340 Inventor(s): D AMELIO FRANK SR (US); MIRHOM YOUSSEF (US) Applicant(s): BIO BOTAN INC (US); D AMELIO FRANK SR (US); MIRHOM YOUSSEF (US) IP Class 4 Digits: None IP Class:None Application Number: WO2005US06778 (20050303) Priority Number: US20040549155P (20040303) Family: US2005196475 Equivalent: WO2005084340; US2005196475A1; WO2005084340A2; WO2005084340A3 Cited Document(s): US6352685; US6673377; XP002954065 Abstract: Abstract of WO2005084340 An extract is derived from Pueraria candollei var. mirifica A Shaw. & Suvat. for topical and internal use in treating a subject. The extract is obtained by a sequential extraction process using a sequence of different solvents or mixtures of solvents in varying ratios and thereafter combining the liquid extracts. The liquid extract is then evaporated to dryness to obtain the extract. The extract is used in a topical composition that when applied to the skin reduces wrinkles without eye irritation.Description: >;Desc/Clms Page number 1; PUERARIA CANDOLLEI VAR. MIRIFICA A SHAW. & SUVAT. EXTRACT Field of the Invention [0001] The present invention is directed to a process for obtaining an extract of Pueraria candollei var. mirifica A Shaw. & Suvat. and to the extract obtained by the extraction process. The invention is also directed to an extract of Pueraria candollei var. mirifica A Shaw. & Suvat. that contains miroestrol with lesser amounts of isomers and derivatives of deoxymiroestrol, genistein, diadzin, puerarin, genistin and diadzein in effective amounts for topical and internal use. Background of the Invention [0002] For more than500 years, people in South East Asia have been using the root of"White Kwao Krua"for its profound anti-aging properties. This root has been identified asPueraria candollei var.mirifica Airy Shaw et Suvat, which belongs to the family Papilionaceae (Leguminosae). [0003] Folklore states that the root is a"fountain of youth"for aged men and women. It serves as an anti-wrinkle agent for aged and wrinkled skin, darkens white hair and increases hair growth, alleviates cataract problems, helps with memory loss, increases energy and vigor, blood circulation, appetite and sleep disorders. [0004] The name mirifica in Latin means"miracle". There are more than 13 related, identified species of Pueraria with similar tuberous roots. They can be differentiated by thetaxonomical characteristics of Mathéo Software (c) http://www.matheo-software.com 46/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) leaf, flower, inflorescence, fruit and covering trichomes. The only species with distinct estrogenic activity was found to bePueraria candollei var.mirifica A. Shaw.8n Suvat.(hereinafter"Pueraria mirifica or P. mirifica). Even the taxonomically similar speciesPueraria >;Desc/Clms Page number 2; candollei Graph. Ex. Benth. does not contain any measurable amount of Miroestrol and also lesser amounts of isoflavonoids. [0005] Therefore, the properly identified roots are collected and their Phytoestrogens HPLC fingerprints authenticated before any further processing. For best results, the location, age of plant (2-4 years), atmospheric conditions (no rain), drying process and storage conditions are carefully monitored. [0006] In"Anusarnsoondhorn, L. (1931) The Ingredientof PuerariaTuberous Root", translated from Siamese, it is mentioned that the use of this root will make the skin smooth like a six year old child and allow you to live 1,000 years and prevent suffering from parasites,while also enhancing memory. Wanadorn, P. W. , in"A Reputed Rejuvenator", J. Siam Society, Natural History Supp., 8, 337(1931), wrote"The ability of P. mirifica to produce a soft, youthful skin, and to turn White hair black, is stressed". Summary of the Invention [0007] The present invention is directed to a process for obtainingan extract of Pueraria candollei var. mirifica A Shaw. & Suvat. , the extract obtained by the process and the composition containing the extract. The invention is further directed to methods of treating a subject by administering topically or internally. [0008] One aspect of the invention is to provide a process for reducing the wrinkles on the skin substantially without eye irritation. In one embodiment, the topical composition contains about 0.5 to about 3.0 parts by weight of the extract which is applied topically to the target site. [0009] The extract composition of the invention contains substantially all of the extractable components from the plant material in a concentrated form. The extract in preferred embodiments contains the extractable components in substantially the same ratio as in the original plant material. Preferably, the extract contains miroestrol, deoxymiroestrol, diadzin, puerarin, genistin, diadzein and genistein. [0010] The extract of the invention is preferably carried out by a sequential process where the plant material is contacted in successive steps with a solvent. The solvent in each successive step is preferably an aqueous solvent containing a water miscible organic solvent in different ratios. In one embodiment, the successive solvents contain increasing amounts of the organic solvent. Each successive extraction solvent preferably increases the amount of the organic solvent by at least 5%, and typically about 5-10% by volume. In one particular embodiment, the extraction process utilized three successive extraction steps where each successive extraction liquid is an aqueous liquid containing 40%, 50% and 60% ethanol, respectively. [0011] The various embodiments of the invention are basically attained by providing a process for producing an extract ofPuerariacandollei var. mirifica Airy Shaw.& Suvat. comprising the steps of: obtaining a dry particulate ofPueraria candollei var. mirifica A Shaw. & Suvat.; contacting the particulate with an aqueous solvent including about 40% to about 60% by volume of a lower alcohol, and obtaining a liquid extraction; and drying the liquid extraction to obtain a substantially dry extract. [0012] The advantages of the invention are further attained by providing a composition having an antiskin wrinkling effect to the skin of a subject without irritation to the eye of the subject. The Mathéo Software (c) http://www.matheo-software.com 47/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) composition comprises a carrier and an effective amount of an extract of Pueraria candollei var. mirifica A Shaw. & Suvat. to reduce skin wrinkles. The extract is obtained by the process of obtaining a dry particulate of Pueraria candollei var. mitifica A Shaw. & Suvat. , contacting the particulate with a first aqueous extraction liquid containing ethanol and obtaining a first liquid extract, and thereafter contacting the particulate with a second aqueous extraction liquid containing ethanol and obtaining a second liquid extract. The ethanol concentration of the second extraction liquid is greater than the ethanol concentration of the first extraction liquid. The first and second extraction liquids are combined and evaporated to dryness to obtain the extract. [0013] The advantages of the invention are also attained by providing a process of reducing wrinkles of the skin of a subject in need thereof comprising topically applying a composition containing an effective amount of an extract ofPueraria candollei var. mirifica A Shaw.dz Suvat. to the skin of a subject to reduce wrinkles of the skin, wherein the extract is obtained according to the process of the invention. [0014] The advantages of the invention are still further attained by providing a substantially dry extract of Pueraria candollei var. yrnn/zca A Shaw. 8s Suvat. obtained by the process of obtaining a dry particulate of Pueraria candollei var. mirifica A Shaw. dz Suvat. and contacting the particulate with a first aqueous solvent including about 40% to about 49% by volume of a lower alcohol and obtaining a first liquid extraction. The particulate is contacted with a second aqueous solvent including about 50% to 59% by volume of a lower alcohol and a second liquid extraction is obtained. The particulate is contacted with a third aqueous solvent including about 60% to 69% by volume of a lower alcohol and a third liquid extraction is obtained. The first, >;Desc/Clms Page number 5; second and third liquid extractions are combined and the extractions are dried to obtain a substantially dry extract. [0015] These and other aspects of the invention will become apparent from the following detailed description of the invention. Detailed Description of the Invention [0016] The present invention is directed to an extract and to a process for producing an extract fromPueraria candollei var. mirifica A Shaw.& Suvat. The resulting extract is found to have all of the active compounds present in the native plant material. For example, the extract contains a concentrated mixture of the phytoestrogens in substantially the same ratio as in that are found in the plant. [0017] A primary aspect of the invention is directed to an extraction process, the resulting extract and compositions containing the extract obtained fromPuerariacandollei var. mirifica Airy Shaw.dz Suvat. [0018] The genus Pueraria comprises numerous members, which are widespread in Asia, Australia, Africa and North, Central and South America. Thirteen species have been taxonomically identified in Thailand, namely: 1. Pueraria candollei Graph. Ex. Benth 2. Pueraria candollei var. mirifica A Shaw. dz Suvat. 3.Pueraria alopecuroides Craib 4.Puerariaimbricata van der Maesen sp. Nov. 5.Pueraria lobata6. Pueraria lobata var. ontana 7.Pueraria lobata var. thomson 8.Pueraria var. phaseoloides 9.Pueraria var. javanica 10.Pueraria var. subspicata Mathéo Software (c) http://www.matheo-software.com 48/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) >;Desc/Clms Page number 6; 11.Pueraria wallichii 12. Pueraria rigens 13.Pueraria stricta [0019] The required species of the present invention is not"Puerariarnirifica"but Pueraria candollei var. mirifica A Shaw.& Suvat. WhilePueraria candollei Graph Ex. Benth is closely related, it takes highly skilled harvesters to be able to select the correct species.Puerariacandollei Graph Ex. Benth does not contain Miroestrol, which is the desired constituent of the present invention. In addition, the isoflavonoid content is much lower inPueraria candollei Graph Ex. Benth than inPueraria candollei var. mirifica A Shaw.& Suvat. Collectors should avoid collecting any of the other 11 species widespread in Thailand. [0020] The extract is obtained by a solvent extraction process from the tuberous roots of Pueraria candollei var. mirifica A Shaw. & Suvat. In one embodiment, the tuberous roots are dried and ground to a powder having a particle size that is amenable for solvent extraction. In other embodiments, the raw plant material is used without drying. The resulting powder or macerated plant material is placed in a column or filtration device and the solvent is passed through the column or filtration device. The volume of the solvent is selected in relation to the amount of the plant material being treated, and is preferably sufficient to extract substantially all of the extractable compounds from the plant material. The extraction solvent can be continuously passed through a bed of the powdered or granulated plant material. Alternatively, the extraction solvent can be contacted with the powder or granular plant material in several batches and then the batches can be combined. >;Desc/Clms Page number 7; [0021] In preferred embodiments, the extraction solvent containing the extracted compounds is evaporated to produce a substantially dry alcohol free extract. The solvent can be recovered for further use, if desired. Preferably, all or substantially all of the extraction solvent is evaporated to provide a substantially dry solid extract containing all of the extractable compounds, and preferably substantially all of the phytoesterols from the plant material. Preferably the resulting extract is a free flowing powder. In one preferred embodiment, the extract contains substantially all of the miroestrol, and deoxymiroestrol that were present in the native plant material and in the same proportions of the native plant material. [0022] Preferably, the solvent is removed by an evaporation process that does not cause decomposition of the active components that are extracted from the plant material. In one embodiment, the solvent is removed under a pressure below atmospheric pressure and at a temperature that is sufficient to remove the solvent efficiently without decomposing the extracted compounds. In a preferred embodiment, the solvent is evaporated at a temperature of about45 C. [0023] The extraction solvent can be one or more of a number of suitable solvents as known in the art. Examples of solvents that can be used include water, ethanol, methanol, propanol, isopropanol, hexane and lower aldehydes and ketones, as well as other organic solvents as known in the art. The extraction process can use one or more solvents that can be combined to form a single solvent system. Preferably, the extraction solvent is an aqueous solvent containing about 40% to about 60% by volume of a lower alcohol, which is preferably ethanol. Alternatively, the solvents can be used separately to obtain different extracts containing a fraction of compounds that are extracted by the particular solvent. The separate extracts are then Mathéo Software (c) http://www.matheo-software.com 49/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) >;Desc/Clms Page number 8; combined and the solvent evaporated to obtain the dry extract. The solvent and cosolvents are preferably miscible with each other when the extraction system contains a mixture of solvents. [0024] In one preferred embodiment, the extraction solvent is in a water-ethanol mixture. The concentration of the ethanol in one embodiment ranges from about 40% to about 60% by volume. In other embodiments, the extraction process is carried out using successive extraction steps with an extraction solvent containing different ratios of ethanol and water. Each of the resulting extract solutions are combined and the solvent evaporated to obtain the dry extract. One embodiment of the invention uses successive extraction steps containing successively increasing amounts of ethanol. For example, the successive extraction steps use a water-ethanol mixture in a ratio of about 40% by volume ethanol to about 60% by volume ethanol in 5% increasing increments. In other embodiments, the extraction process uses three extraction steps using water-ethanol mixtures containing 40%, 50% and 60% by volume ethanol for each successive extraction step. In each of these processes, the extraction solvents are combined and evaporated to produce the dry extract. Alternatively the extraction process can be carried out in successive steps using decreasing amounts of the alcohol in the aqueous solvent. [0025] In one preferred embodiment, the plant material is extracted with successive aqueous solvent mixtures containing at least 40% by volume of a lower alcohol and particularly ethanol. The extraction contacts the plant material with a first extraction liquid containing about 40% to about 49% of an alcohol to obtain a first liquid extract. Thereafter, the plant material is subsequently contacted with a second aqueous extraction liquid containing about 50% to 59% of an alcohol to obtain a second liquid extract. The plant material in one preferred >;Desc/Clms Page number 9; embodiment is then contacted with a third extraction with a third extraction liquid containing about 60 to about 65% of an alcohol where the percentages are by volume. In a further embodiment, the third extraction liquid can contain about 60% to about 69% of the alcohol. The liquid extracts are then combined and reduced to dryness to obtain a powdered extract. Preferably the same alcohol is used in each of the extraction steps. [0026] The resulting extract is preferably alcohol free and can be reduced to a free flowing powder or granular material. The powder can then be dispersed in a suitable carrier for topical or internal use. The powder can also be used to form capsules or tablets of the substantially pure extract for internal use. [0027] In certain embodiments, the extract obtained from thePuerariacandollei var. mirifica A Shaw.& Suvat. is combined with a suitable solvent or carrier. Examples of suitable carriers include butylene glycol, propylene glycol and glycerin. Glycerin is generally preferred for topical and internal use. The carrier can contain small amounts of water. Preferably, the amount of water is sufficiently small to inhibit the precipitation of compounds that are insoluble or only marginally soluble in water and to inhibit the growth of microorganisms. In one embodiment, a topical composition is a suspension consisting essentially of glycerin and about 1.0 to 5.0% by weight of the dry powdered extract. [0028] In preferred embodiments, the final composition contains at least about 20 mg miroestrol and deoxymiroestrol per 100 g of the composition. The composition also contains the various isoflavonoids that are present in the plant material. The isoflavonoids are preferably included in the composition to provide about 3-11 mg diadzin, about 12-30 mg puerarin, about 0.5-2 mg genistin, about 1.1 >;Desc/Clms Page number 10; to 3.6 mg diadzein, and about 0.2 to 2 mg genistein based on 100 g of the final composition. Mathéo Software (c) http://www.matheo-software.com 50/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) [0029] The extract ofPueraria candollei var.mirifica AShaw. & Suvat. is used to produce various topical compositions for cutaneous use and oral compositions for internal use. Generally, the final composition contains about 0.5 to about 3.0 w/w of the extract. The topical compositions are suitable for use in applying to the breast to promote breast enlargement. In other embodiments, the composition is applied to the skin of a subject in need thereof to reduce the appearance of age lines and wrinkles, particularly around the eyes. The extract can be used around the eyes to reduce the signs of wrinkles without irritation of the eyes since the extract is non- irritating to the eyes. The topical compositions are generally in the form of body gels, creams and lotions. [0030] In another embodiment, the extract ofPueraria candollei var. mirifica A Shaw.& Suvat. is administered orally to a patient in need thereof as an estrogen replacement for menopausal women. It is believed that the phytoestrogens of the extract ofPueraria candollei var.mirificcc A Shaw.dz Suvat. bind to the estrogen receptors with respective degrees of affinity. In this manner, the phytoestrogens from the extract ofPueraria candollei var. mirificaA Shaw. & Suvat. can reduce the effects of estrogen insufficiency in the body and provide relief to the symptoms caused by estrogen insufficiency. The consumption or internal use of the phytoestrogen present in the extract can also lower the risk of cardiovascular diseases that can result from conventional estrogen replacements. Isoflavonoids similar to those extracted fromPuerariacandollei var. mirifica A Shaw.& Suvat. are known to decrease the level of LDL cholesterol and increase the level of HDL in blood. The extract ofPueraria candollei var. >;Desc/Clms Page number 11; mirifica A Shaw.& Suvat. is administered orally to a subject in need thereof in a method to reduce the level of low density lipids (LDL) and increase the level of high density lipids (HDL) in humans. [003 1] Puera7ia cand ollei var. mirifica A Shaw. & Suvat. contains different types of phytoestrogens, which may be broadly classified into three main groups: 1. The miroestrol and deoxymiroestrol are comparatively potent phytoestrogens unique to Pueraria candollei var. mirifica A Shaw. & Suvat. , their structural configuration is very close to the structural configuration of estradiol-17 as shown by the following formulas. EMI11.1 HO 13, OH , x OH ? u a r,.,. OH, OH OH i I, 4 6 li 3 I6 17 O 7 OFI r I I /I q 8 ' i s \ 6 Fi O'3 \,. H Miroestrol Deoxymiroestrol Estradiol-17B 2. The isoflavonoid compoundsof Pueraria candollei var. mirifica A Shaw. & Suvat. , which comprises daidzin and daidzein, genistin and genistein, and puerarin have the formulas: >;Desc/Clms Page number 12; EMI12.1 OH H CH20H H20H 51 OH zozo 0 00 Oh 6 \ I, z 3t 6'\ W"'OH 5-r'OH Puerarin Daidzin R=H ; MW 416 MW 416 Genistin R=OH ; MW 432 H 0 / >;\OR ORAL Diadzein R=H, Rl=H ; MW 254 Genistein R=OH, Riz ; MW 270 3. The coumestan compound comprises coumestrol. [0032] This wide variety of phytoestrogenic compounds belonging to different groups and present side by side in a single plant makesPueraria candollei var. mirifica A Shaw.& Suvat. a very suitable herb for hormone supplements to treat the decline in the natural hormones in women over 45 years of age. Taking estrogenic hormones has been shown to be carcinogenic in some case studies. On the other hand, taking phytoestrogens proved to be anti-carcinogenic or at least delay carcinogenic manifestations. A considerable amount of data has been accumulated proving that phytoestrogens exhibit an inhibitory effect >;Desc/Clms Page number 13; Mathéo Software (c) http://www.matheo-software.com 51/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) against mammary tumorigenesis, prostate-related cancer and bladder cancer. [0033] Phytoestrogens also exhibit favorable effects other than the dramatic results of protection against certain types of cancer. In other words, as a result of the decrease in the level of estrogenic hormones, the body's systems and their functions begin to decelerate and the immune system weakens. Accordingly, menopausal women will usually experience both psychological and physiological changes. Psychological changes range from anxiety, tension and nervousness to chronic depression, whereas the physiological changes include hot flashes, fatigue and insomnia, to more severe conditions such as bone loss, balding and degeneration of the reproductive area. [0034] The structure of miroestrol is not a steroidal hormone but is a 21 carbon compound with a 6membered ring instead of the 5- membered ring of estradiol-17 . The similarity between miroestrol and estradiol-17 is in the size of the 4-ring molecule. The aromatic ring with the phenolic hydroxyl group at position 3 are present in miroestrol and estradiol-17 (3. The (3-hydroxyl group at position 18 in miroestrol corresponds to the 17 (3-hydroxyl group of estradiol-17 and the (3-hydroxyl group in the 17 position of estriol. The 17a-hydroxyl group of miroestrol corresponds to the 16a-hydroxyl group of estriol. Miroestrol is a unique phytoestrogen that is very similar to estriol while being non-hormonal and nonsteroidal. The unique estrogenic effect ofPueraria candollei var. mirifica A Shaw. 8s Suvat. is a result of the similarity of the standardized liquid product of the invention. While being the form of choice for formulating products for cutaneous applications, it is also very suitable for internal use providing a superior bioavailability. The preferred vehicle is glycerol as the only solvent, carrier or vehicle. However, since many people find the solid >;Desc/Clms Page number 14; dosage form practical for administration and accurate dosing, a powdered extract is produced which can be easily formulated into tablets and capsules. [0035] The powdered tuberous root of Pueraria candollei var. inirifica A Shaw. & Suvat. differs in analysis from one season to another and is affected considerably by the atmospheric conditions. In particular, the amount of rain in the region of the world where the plant is indigenous is a significant factor in determining the amount of each of the phytoestrogens in the plant material. Therefore, for internal therapeutic use, the production of a standardized powdered extract is preferred. No such product has been produced so far for the market. [0036] The same identification and extraction procedure is followed for the preparation of the standardized liquid. The extract is analyzed chemically and the percentage of solids determined. The calculated amount of diluent is added. Such diluent may be maltodextrin orCapful, then USP purified water is added to bring the total solids to about 40% which is a suitable concentration for spray-drying. The optimum spray-drying condition forPueraria candollei var. mirifica A Shaw.& Suvat. to obtain a powdered extract is 390-400 F inlet temperature and 190-200 F outlet temperature (regulated by the rate of flow of the feeding liquid). During spray-drying, the relative humidity is kept between 40-50%. In one embodiment of the invention a spray dried powdered extract is obtained by preparing a mixture of a liquid carrier containing about 20% to about 50% and preferably about 35% to about 45% solids of the extract from the plant material and spray drying. The resulting spray dried extract is a dry free flowing powder. [0037] The standardized powdered extract is adjusted to comply with the adjusted product specification which is identical to the concentrations of the liquid extract product. Preferably, each 100 g of the powdered extract contains : 20 mg miroestrol (deoxymiroestrol), about 3-11 mg daidzin; about 12-30 mg puerarin; about 0.5-2 mg Mathéo Software (c) http://www.matheo-software.com 52/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) genistin; about 1.1-3 mg daidzein; and about 0.2-2 genistein. The recommended dose of the product for internal use is 80 mg of the extract 1 to 2 times/day. [0038] The stability of the constituents was monitored by HPLC before and after spray-drying. No decomposition or degradation could be noticed (neither new peaks, nor disappearance nor reduction of any peak, nor the appearance of new shoulders). This provides reasonable assurance that a constituent, standardized and effective product is produced. Toxicity tests using a mutagenicity assay have shown the extract is negative for Staphylococcus Aureus, Salmonella typhimurium and E. coli. Example 1 [0039] This example evaluates the toxicity and irritancy of the extract. The chick embryo has been used extensively in toxicology. The chorioallantoic membrane (CAM) of the chick embryo is a complete tissue with organoid elements from all germ cell layers. The chorionic epithelium isectodermal and the allantoic epithelium is endodermal. The mesoderm located between these epithelia is a complete connective tissue including arteries, capillaries, veins and lymphatic vessels. The CAM responds to injury with a complete inflammatory reaction, comparable to that induced in the rabbit eye test. It is technically easy to study, and is without nerves to sense pain. [0040] Fresh, fertile, White Leghorn eggs were obtained from Avian Services in Frenchtown, New Jersey. They were stored at this facility for up to seven (7) days, at 13 C, before being incubated. For incubation, the eggs were placed on their sides, in a Kuhl incubator. The incubator is such that the eggs are automatically rotated once every hour. The temperature was controlled at99 F(1 ) with a relative humidity of 60-70% for the ten (10) days of incubation. On day eight (8) the eggs were turned so that the acutely angled end faced down. [0041] On day ten (10), each egg was removed from the incubator and placed in a Plexiglas work enclosure. This enclosure had been preheated and humidified so that its environment approached that of the incubator. A cut was made in the larger end of each egg, where the air sack is located. A Dremel Moto-Flex Tool (model 232-5) equipped with aDremel) Cut-Off Wheel (No. 409) was used to make each cut. Forceps were then used to remove the shell down to the shell-membrane junction. The inner egg membrane was then hydrated with a warm, physiological saline solution. The saline was removed after a two (2) to five (5) minute exposure. Utilizing pointed forceps, the inner egg membrane was then carefully removed to reveal the CAM. [0042] The test or reference article, at a dosage of three-tenths of one milliliter (0.3 ml) of a liquid or three-tenths of one gram (0.3 g) of a solid was then administered to each of four (4) CAM's. Twenty seconds later, the test or control article was rinsed from each CAM with five (5) milliliters of physiological saline. All CAM's were observed immediately prior to test article administration and at 30 seconds, two (2) and five (5) minutes after exposure to the test article. The reactions of the CAM, the blood vessels, including the capillaries, and the albumin were examined and scored for irritant effects as detailed below: >;Desc/Clms Page number 17; Score Effect Time (min.) 0. 5 2 5 Hyperemia 5 3 1 Minimal Hemorrhage ("Feathering")7 5 3 Hemorrhage (Obvious Leakage)9 7 5 Coagulation and/or Thrombosis 11 9 7 [0043] The numerical, time dependent scores were totaled for each CAM. Each reaction type can be recorded only once for each CAM, therefore the maximum score per CAM is 32. The mean score was determined for all CAM's similarly tested. Test Article(%) CAM# Scores@ 0.5 min. 2 min. 5 min. Total PM, PE, Product Code: 1 0 0 1 1 4523, Lot Number: 031615 2 0 3 0 3 Date:03/01/04 (2.5%) 3 0 3 0 3 Mathéo Software (c) http://www.matheo-software.com 53/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 40303 Average: 2.50 Reference Article(%) CAM# Scores@ 0.5 min. 2 min. 5 min. Total Johnson's Baby 1 5 7 0 0 12 Shampoo (50%) 2 5 7 0 0 1230 3 5 0 84 5 7 0 0 12 Average: 11.00 Reference Article (%)CAM#Scores@ 0.5 min. 2 min. 5 min. Total Headdz Shoulders 1 5 7 0 5 7 24 >;Desc/Clms Page number 18; Shampoo (50%) 2 5 7 0 5 7 243 5 7 7 0 194 5 7 0 5 17 Average: 21.00 [0044] Each article was then classified as indicated in the following: Mean Score Irritation Potential 0.0-4. 9 Practically none 5.0- 9. 9 Slight 10.0-14. 9 Moderate 15.0-32. 0 Severe[0045] The irritation potential of this test article was at 5%. Previous studies have shown that the CAM of the hen's egg is more sensitive to liquid irritants than is the rabbit eye. Therefore, dilutions of the liquid test and reference articles were used. The Johnson's reference product has historically been categorized as being moderately irritating, eliciting scores approaching 10, at 24 hours, when dosed at 100% and tested using the Draize ocular irritation methodologies. The Head& Shoulder's reference product has historically been categorized as being severely irritating, eliciting scores approaching 30, at 24 hours, when dosed at 100% and tested using the Draize ocular irritation methodologies. Under the conditions of this test, the results indicate that the extract exhibited practically no ocular irritation potential in vivo. [0046] While various embodiments of the invention have been chosen to illustrate the invention, it will be understood by those skilled in the art that various changes and modifications can be made >;Desc/Clms Page number 19; without departing from the scope of the invention as defined in the pending claims.Data supplied from the esp@cenet database - Worldwide Claims: WHAT IS CLAIMED IS: 1. A process for producing an extract ofPueraria candollei var. mirifica Airy Shaw.& Suvat. comprising the steps of: obtaining a dry particulate ofPuerariacandollei var. mirifica A Shaw. & Suvat.; contacting said particulate with an aqueous solvent including about 40% to about 60% by volume of a lower alcohol, and obtaining a liquid extraction; and drying the liquid extraction to obtain a substantially dry extract. 2. The process of claim 1, wherein said lower alcohol is ethanol and where said aqueous solvent consists essentially of water and ethanol. 3. The process of claim 2, further comprising sequentially contacting said particulate with a plurality of aqueous solvents where each of said sequential aqueous solvents contain a different amount of ethanol. 4. The process of claim 2, further comprising contacting said particulate with a first aqueous solvent containing about 40% to about 49% by volume ethanol and obtaining a first extraction liquid; thereafter contacting said particulate with a second aqueous solvent and obtaining a second liquid extract, wherein said second aqueous liquid has an alcohol concentration greater than said first aqueous solvent; and combining said first and second liquid extracts and drying said liquid extracts to a dryness to obtain said extract. >;Desc/Clms Page number 21; Mathéo Software (c) http://www.matheo-software.com 54/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 5. The process of claim 3, wherein said second aqueous liquid contains about50% to 59% by volume ethanol, said process further comprising contacting said particulate with a third aqueous liquid containing about 60% to about65% ethanol to obtain a third liquid extract; combining said third liquid extract with said first and second liquid extracts; and drying said liquid extracts to obtain said extract. 6. The process of claim 1, wherein said extract comprises at least about 20 mg miroestrol and deoxymiroestrol per 100 g of said extract. 7. The process of claim 1, wherein said extract comprises about 3-11 mg diadzin, about 12-30 mg puerarin, about 0.5-2. 0 mg genistin, about1.1-3. 6 mg diadzein, and about 0.2-2 mg genistein based on 100 g of said extract. 8. A composition having an anti-skin wrinkling effect to the skin of a subject without irritation to the eye of the subject, said composition comprising a carrier; and an effective amount of an extract ofPueraria candollei var. mirifica A Shaw.& Suvat. to reduce skin wrinkles, wherein said extract is obtained by the process of obtaining a dry particulate ofPueraria candollei var.mirifica A Shaw. & Suvat.; contacting said particulate with a first aqueous extraction liquid containing ethanol and obtaining a first liquid extract; >;Desc/Clms Page number 22; thereafter contacting said particulate with a second aqueous extraction liquid containing ethanol and obtaining a second liquid extract, wherein an ethanol concentration of said second extraction liquid is greater than the ethanol concentration of said first extraction liquid; combining said first and second extraction liquids and evaporating to dryness to obtain said extract. 9. The composition of claim 8, wherein said composition contains about 0.5 to about 3 parts by weight of said extract. 10. The composition of claim 8, wherein said first aqueous extraction liquid contains about 40% to about 49% by volume ethanol. 11. The composition of claim 10, wherein said second aqueous extraction liquid contains about 50% to about 59% by volume ethanol. 12. The composition of claim 11, wherein said process further comprises contacting said particulate with a third aqueous extraction liquid containing about 60-65% by volume ethanol and obtaining a third extraction liquid, and combining said first, second and third extraction liquids and evaporating to dryness to obtain said extract. 13. The composition of claim 8, wherein said first and second extraction liquids consist essentially of water and ethanol. >;Desc/Clms Page number 23; 14. The composition of claim 8, wherein said extract comprises about 15-25 mg miroestrol and deoxymiroestrol, about 3- 11 mg diadzin, about 12-30 mg puerarin, about 0.5-2. 0 genestin, about1.1-3. 6 mg diadzein and about 0.2-2 mg genistein based on 100 g of said extract. 15. A process of reducing wrinkles of the skin of a subject in need thereof comprising: topically applying a composition containing an effective amount of an extract of Puerariacandollei var. mirifica A Shaw.dz Suvat. to the skin of a subject to reduce wrinkles of the skin, wherein said extract is obtained according to the process of claim 1. 16. The process of claim 15, comprising applying said composition to the skin around the eyes of said subject to reduce wrinkles around the eye without irritation to the eye. Mathéo Software (c) http://www.matheo-software.com 55/56 เอกสารสิทธิบัตรยุโรป จากฐานข้ อมูล http:///gb.espacenet.com เรื่อง กวาวเครือขาว (Pueraria mirifica) 17. The process of claim 15, wherein said extract comprises about 15-25 mg miroestrol and deoxymiroestrol, about 3-11 mg diadzin, about 12-30 mg puerarin, about 0.5-2. 0 genestin, about 1.13.6 mg diadzein and about 0.2-2 mg genistein based on 100 g of said extract. 18. The process of claim 15, wherein said composition contains about 0.5 to about 3 parts by weight of said extract. 19. A substantially dry extract ofPueraria candollei var.mirifica A Shaw.& Suvat. obtained by the process of >;Desc/Clms Page number 24; obtaining a dry particulate ofPueraria candollei var.mitift-ca A Shaw.& Suvat.; contacting said particulate with a first aqueous solvent including about 40% to about 49% by volume of a lower alcohol and obtaining a first liquid extraction; contacting said particulate with a second aqueous solvent including about 50% to 59% by volume of a lower alcohol and obtaining a second liquid extraction; contacting said particulate with a third aqueous solvent including about 60% to 65% by volume of a lower alcohol and obtaining a third liquid extraction; and combining said first, second and third liquid extractions and drying said extractions to obtain a substantially dry extract.Data supplied from the esp@cenet database - Worldwide Mathéo Software (c) http://www.matheo-software.com 56/56