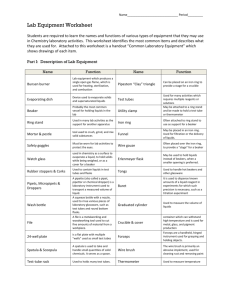
The Bunsen burner is the most commonly used source of heat the in
advertisement

Using a Lab Burner Handout # Remember to embed questions in the prelab and postlab (Analysis and Conclusions) responses. Introduction The lab burner is one of the most efficient methods to heat materials in the chemistry classroom. The burner is connected to a natural gas supply on your lab table. In this lab, you will learn the parts of a Tirrill burner and their functions. You will also learn how to use your burner safely. Observe all safety precautions necessary for working with open flames in the science lab. All lab partners must perform each part of this lab. Materials Needed Tirrill burner and tubing flint striker copper wire crucible tongs ruler 250 mL beaker thermometer 100-mL graduated cylinder water Safety Wear a laboratory apron and safety glasses. Tie back long hair and loose clothing. Use extreme caution when working with heated materials to avoid burns. Whenever your burner is not in use, make sure the gas valve on your lab table is shut off. Procedure: Part 1 1. Without connecting your burner to the gas outlet, examine your lab burner. Take apart your lab burner and reassemble it. Locate the following parts: The barrel is where the air and gas mix. The collar is at the base of the barrel and has adjustable air ports. If you turn the collar so that the holes are larger, more air will be drawn into the barrel. The base supports the burner so that it does not tip over. The spud is an adjustable needle valve used to regulate the flow of gas into the burner. 2. Note the gas valve on the lab table. If the handle of the valve is parallel to the burner tubing, the gas flow is at a maximum. If the handle is perpendicular to the burner tubing, the gas flow is off. Lab Burner Lab 3. Reassemble the burner and connect the burner tubing to the gas valve. CAUTION: Put on your safety goggles and leave them on for the rest of the lab. 4. Prepare your burner for lighting. Adjust the air ports so that they are slightly open (<50%). Adjust the spud for full gas flow. 5. Hold the flint striker about 5 cm above the top of the barrel, turn the gas valve completely on, and produce a spark. This will cause the gas coming out of the barrel to ignite. CAUTION: If the flame is burning from beneath the barrel, immediately turn the gas off at the jet. Allow the burner to cool. Repeat step, only reduce the amount of air entering the barrel. 6. If your burner is adjusted properly, the barrel will remain cool and the flame will be blue. If the flame is yellow, this means the gas is not burning completely and carbon monoxide, a poisonous gas, is being produced. Adjust the air ports to produce a blue flame with an inner blue cone. 7. Then adjust the gas valve to produce a flame that is 6 cm high. Have your instructor initial this step. 8. Make sure each member of your lab group performs steps 4, 5, 6 and 7. 9. Completely close the air ports and record what happens to the flame. Data & Observations: (Leave space to complete observations from Part 1. This should include any difficulties encountered in producing the flame – flickering, etc., as well as your response to # 9. ) Part 2 1. Using crucible tongs to hold an evaporating dish at the tip of the flame from Step 9 in Part 1. 2. Carefully place the evaporating dish on the lab table. CAUTION: Hot glassware is more fragile than cool glassware and breaks easily. 3. After the dish has cooled, observe the underside of the dish. Record your observations. 4. Adjust the gas so that the flame extends about 8 cm above the barrel. 5. Adjust the air vents so that you have a steady blue flame with a sharply defined inner blue core. This adjustment gives you the hottest possible flame. 6. Using the crucible tongs hold a 10-cm piece of copper wire in the flame just above the barrel. Slowly lift the wire up through the flame until you locate the hottest part of the flame. Where is the hottest part of the flame located? 7. Hold the wire in the hottest part of the flame and describe what happens. 8. Shut off the burner by turning off the gas valve. 9. When the materials are cool, put them away. Return the copper wire to the supply station. Data and Observations: (Leave space to complete observations from Part 2. This should include your response to # 3, as well as a description of the copper wire before you put it in the flame, as it is in the flame, and after you remove it from the flame.) Part 3 1. Measure approximately 75 mL of warm water from your instructor in the graduated cylinder. Record the volume of water to the nearest tenth of a milliliter. 2. Put a thermometer in the cylinder and record the temperature to the nearest tenth of a degree. 3. Pour the water into a 250-mL beaker. 4. Light your burner and adjust it so the hottest part of the flame is on the bottom of the wire mesh directly under the beaker. 2 Lab Burner Lab 5. Place the beaker on the wire mesh. You will need to record the time needed to bring the water to a boil, so be sure to note the time (including seconds) that you began heating the water. 6. Watch the beaker carefully until the water is boiling. If large bubbles are rising from the bottom of the beaker to the surface, the water is boiling. NOTE: Small bubbles that stay at the bottom or sides of the beaker are air bubbles present in the water; they do not mean that the water is boiling. Wait for bubbles that rise to the top of the beaker. Record the time in minutes and seconds that this occurs. Carefully hold the thermometer in the water to record the temperature of boiling water. Do not let it touch the bottom. Do not burn your hand! Remove the thermometer. 7. Continue boiling for 2 minutes and record this temperature as Tf.. 8. Turn off the gas. 9. Using beaker tongs or the hot vessel gripper, carefully remove the beaker from the ring stand and pour the hot water into the sink. Then gently place the beaker on the lab table to cool. When it is cool, wash and dry the beaker. 10. Complete the data table Data and Observations Fill in the data table in your lab books and record any other pertinent observations. Part 3 Data Table Volume water Initial temperature (Ti) Boiling temperature (Tb) Time required boil water. Final Temperature after 2 minutes of boiling (Tf) Analysis and Conclusions: 1. Suggest two possible reasons the burner would not light after the gas outlet is opened. 2. What would happen to the flame if the air intake openings were made very small? (1 pt) 3. Where is the hottest part of the flame of a Bunsen burner? (1 pt) Include a sketch of the burner and flame, indicating where the hottest part. (5 pts) 4. Suggest two reasons a blue flame is preferred over a yellow flame in a Bunsen burner. 5. What is the substance that appeared on the bottom of the evaporating dish after it was heated and where did it come from? (2 points) 6. What factors affected the time required to boil the water? Be specific. (2 pts) 7. Did the temperature of the boiling water change over 2 minutes? Can you suggest a reason for your answer? Prelab Questions:. 1. What safety considerations must you address in this lab? (4 pts) 2. What should you do if flames are coming from the base of the Bunsen burner? (1 pt) 3. Why should you tie back long hair? (1 pt) 4. How should you adjust the burner before you put the evaporating dish in the flame? Be very specific. Tell what step in the lab tells you how to adjust the flame. (2) 5. How do you intend to determine the time required to boil the water in Part 3? Be specific. 3