Lab_I_Procedures_and_Safety 2014-2015
advertisement

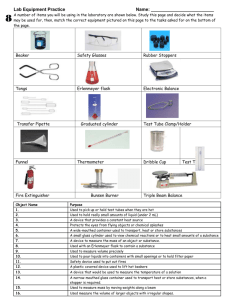
Honors Chemistry Lab 1: LABORATORY PROCEDURES – Lab Safety and Techniques Name ___ Period_______________ Dates_______________ Please note: This lab is a one week lab worth 85 points. It will be graded for completion and accuracy. It is due on Tuesday, September 2nd, many of the questions that are not readily apparent when conducting the lab are answered in the A Special Message on Safety packet. This is the only lab that you will not put in a lab notebook. Instead, after it is graded and returned, you should save it in your binder and use it as a reference Introduction: The best way to become familiar with chemical apparatus is to actually handle the pieces yourself in the laboratory. This experiment is divided into several parts in which you will learn how to adjust the gas burner, use a balance, handle solids, measure liquids, filter a mixture, and measure temperature and heat. Great emphasis is placed on safety precautions that should be observed whenever you perform an experiment and use this apparatus. Several useful manipulative techniques are also illustrated throughout this lab and in A Special Message on Safety. In many of the later experiments, references will be made to these sketches and procedures. You will also be referred to many of the safety precautions and procedures explained in all parts of this experiment. It is important that all students develop a positive approach to a safe and healthful environment in the laboratory. OBJECTIVE After completing this experiment, you should be able to use laboratory equipment safely and skillfully. Safety Take the necessary safety precautions before beginning each part of this experiment. Always wear safety goggles, apron and closed-toe shoes when in the laboratory. Get into the ‘good habit’ of always putting on this standard safety equipment as soon as you enter the lab. It is important that you and your partner observe all safety precautions while conducting experiments. Read all safety precautions while conducting experiments. PART 1 THE BURNER Apparatus Heat Resistant Mat Crucible tongs Burner Evaporating dish Materials- located in hood Matches Copper wire, 18 gauge Special message on safety: In addition to goggles, aprons, and closed toe shoes, all long hair must be tied back, and loose clothing removed or secured out of the way when using a burner. Be sure to follow instructions and do not leave the gas jet on when burner is not lit. Procedures 1. The Bunsen or Tirrell burner is commonly used as a source of heat in the laboratory. Although the details of construction vary among burners, each has a gas inlet located in the base, a vertical tube or barrel in which the gas is mixed with air, and adjustable opening or ports in the base of the barrel. These ports admit air to the gas stream. The burner may have an adjustable needle 1 valve to regulate the flow of gas. In some models the gas flow is regulated simply by the flow of gas. The burner is always turned off at the gas valve on the supply line, never at the needle valve. Look at Figure 1-1 as you examine your Bunsen burner and locate these parts. You must know what happens to the flame when you increase or decrease the gas and when you open and shut the air intake. Be sure to take note what happens to the flame as you adjust your burner. Also, all lab partners must be able to light the burner and adjust the burner, so everyone should practice. Figure 1-1 CAUTION Before you light the burner, check to see that you and your partner have taken the following safety precautions against fires: Wear safety goggles, and aprons. Confine long hair and loose clothing: Tie long hair back at the back of the head away from the front of the face, roll up long sleeves on shirts, blouses and sweaters away from the wrists. You should also know the locations of fire extinguishers, fire blanket, safety showers, and sand buckets and how to use them in case of a fire. 2. In lighting the burner, partially close the ports at the base of the barrel, light the match. Hold the lit match just below the top of the barrel. Turn the gas full on and slowly raise the lit match toward the top of the barrel. The gas flow may be regulated by adjusting the gas valve until the flame has the desired height. If a very low flame is needed, remember that the ports should be partially closed when the gas pressure is reduced. Otherwise, the flame may burn inside the base of the barrel. When improperly burning in this way, the barrel will get very hot, and the flame will produce a poisonous gas, carbon monoxide. Special note: Carbon monoxide is a poisonous gas. If the flame is burning inside the base of the barrel, immediately turn off the gas at the gas valve. Do not touch the barrel of the burner, for it is extremely hot! Allow the barrel of the burner to cool off and then proceed as follows: Begin again, but first decrease the amount of air admitted to the burner by partly 2 closing the ports. Turns the gas full on and then relight the burner. Control the height of the flame by adjusting the gas valve. By taking these steps, you should acquire a flame that is burning safely and is easily regulated throughout the experiment. 3. Once you have a flame that is burning safely and steadily, you can experiment by completely closing the holes (ports) at the base of the burner. Describe what the flame looks like when the ports are completely closed. 4. Using the crucible tongs, carefully hold an evaporating dish in the tip of the flame (underside to the flame) for about 3 – 5 minutes. Place the dish on the ceramic pad, allow the dish to cool, and then examine the underside. Describe the results and suggest a possible explanation for this observation. Such a flame is seldom used in the laboratory. For laboratory work, you should adjust the burner so that the flame will be free of yellow color, nonluminous, and also free from the ‘roaring’ sound caused by admitted too much air. 5. Regulate the flow of gas to give a flame extending roughly 8cm above the barrel. Now adjust the supply of air until you have a quiet, steady flame with a sharply defined, light blue inner cone. This adjustment gives the highest temperature possible with your burner. Using the crucible tongs, inset a 10-cm piece of copper wire into the flame just above the barrel. Lift the wire slowly up through the flame. Where is the hottest portion of the flame located? 6. Hold the wire in this part of the flame for a few seconds. Observe both the flame and the wire. Describe the results of each. 7. Shut off the gas burner. Now think about what you have just observed in procedures 4-6. Why is the nonluminous (blue) flame preferred over a yellow luminous flame in the laboratory? 3 8. Each lab partner needs to practice lighting the burner. If during the course of this procedure each person did not have a turn. Take a moment and allow the other lab partners to practice lighting the burner. Also, if time allows, heat the bottom of the soiled evaporating dish in a nonluminous flame to clean it. (It will be very difficult to clean it by washing, so if necessary, put it away and clean by heating another day.) 9. At the end of this part of the experiment, all equipment you store in the lab locker or drawer should be completely cool, clean, and dry. It should also be arranged in an orderly fashion for the next lab experiment. Check to see that the valve on the gas is completely shut off. Remember that hands should always be washed thoroughly with soap at the conclusion of each laboratory period. PART 2 HANDLING SOLIDS Apparatus Test Tube Spoon Materials – in hood Magnesium Sulfate MgSO4 Weigh paper CAUTION Do not touch chemicals with you hands. Some chemical reagents readily pass through the skin barrier into the bloodstream and can cause serious health problems. Some chemicals are extremely corrosive. Always wear gloves, apron, and safety goggles when handling chemicals. Carefully check the label on the reagent bottle or container before removing any of the contents. Never use more of a chemical than directed. You should also know the locations of the lab shower and eyewash and how to use them in case of an accident. CAUTION Never try to pour a solid from a bottle into a test tube. Never try to put chemicals into a test tube with a spoon. The chemicals could miss and touch your hands, or you would need to clean them up of the table. As a precaution against contamination, never pour unused chemicals back into the reagent bottles. CAUTION Never discard broken glassware into the wastepaper basket. This is an important safety precaution, and it prevents personal injuries to anyone who empties the wastepaper basket. All glass must be discarded in the broken glass container. 4 Never discard chemicals in the wastepaper basket or sink unless told by your teacher to do so. This is an important safety precaution against fires and toxic waste being disposed of inappropriately. Discard chemicals in the proper containers in the hood, unless instructed to do something else. 1. Solids are usually kept in wide-mouthed bottles. A porcelain or plastic spoon should be used to dip out the solid. See figure 1-2. 2. Remove a small spoonful of magnesium sulfate from its reagent bottle. In order to transfer the magnesium sulfate to a test Figure 1-2 tube, first place it on a piece of weigh paper about 10 cm square. Roll the paper into a cylinder and slide it into a test tube that is lying flat on the table. When you lift the tube to a vertical position and tap the paper gently, the solid will slide down into the test tube. 3. Give two reasons why it is important to use this method of transferring solids into a test tube. 1. ________ 2. 4. Set the magnesium sulfate aside, and continue on with Part 3. PART 3 THE BALANCE Apparatus Electronic Balance Spoon Materials – in hood Magnesium Sulfate MgSO4 (from part 2) Weigh paper Procedures 1. When a balance is required for determining mass, we will most often use an electronic balance (shown to the left). Depending on the accuracy required we will use either an electronic balance that records mass to 0.01 or 0.001 Each lab station is also equipped with a centigram balance (shown to the right). The centigram balance is sensitive to 0.01 gram. 2. The centigram balance should only be moved by grasping under the carriage base center support, and gently lifting it off of the table. NEVER grab the balance by the beams. The electronic balance and supporting them from the bottom. 5 3. Before using the centrigram balance, always check to see if the pointer is resting at zero. If the pointer is not at zero, check the slider weights. If all the slider weights are at zero, turn the zero adjust knob until the pointer rests at zero. The zero adjust knob is usually located at the far left end of the balance beam. Whenever weighing chemicals, always use weighing paper or a glass container. 4. NEVER place chemicals or hot objects directly on the balance pan. They can permanently damage the surface of the balance pan and affect the mass weighing 5. Thanks to a generous grant, we have 5 electronic balances to use in our honors chemistry lab. You will find electronic balances more convenient to use than the centigram balance. CAUTION Do not touch chemicals with you hands. Always wear safety goggles and apron when handling chemicals. Carefully check the label on the reagent bottle or container before removing any of the contents. Never use more of a chemical than directed. You should know the locations of the safety shower and eyewash and how to use them in case of an accident. Never place hot glass or chemicals in your lab drawer as this could cause a fire. 6. Place the electronic balance on a flat, stable surface, and check to see that it is plugged in. The precision of the balance relies on minute factors and wind, shaky surfaces, or similar forces will cause the readings to be inaccurate. 7. Press the "ON" button and wait for the balance to show zeroes on the digital screen. 8. Obtain a piece of weigh paper and place it on the balance pan. Record the mass of the weigh paper to the nearest 0.01g. ____________ 9. Using a spoon, or by carefully pouring, add the magnesium sulfate you set aside from PART 2 on to the piece of weigh paper that is on your balance pan. Record the mass.___________ 10. What is the mass of the magnesium sulfate you have? __________ 11. Remove the weigh paper and magnesium sulfate and set it aside. 12. Ensure your balance reads zero. 13. Place a new piece of weigh paper on the balance platform. 14. Press the "Tare" or "Zero" button to automatically deduct the weight of the container from future calculations. The digital display will show zero again, indicating that the container's mass is stored in the balance's memory. 6 15. Remove the weigh paper and notice the reading in the digital display. Place the weigh paper back on the balance pan, notice the reading on the digital display. Describe what is going on?_______________________________________________________________ 16. With the weigh paper on the balance pan, carefully add the magnesium sulfate to the weigh paper, until you have 5 grams. 17. Throw the magnesium sulfate into the labeled waste container in the hood. All weigh paper should be thrown into the regular garbage cans. Clean off your lab table. Note, some lab procedures will ask you to mass the container and record that value, and then record the mass of the container and the substance, and record that value as we did in steps 8-10. If this is the case you would not use the “Tare” (Using the Tare feature allows you to know the mass of the substance without doing any mathematical calculations. This is a helpful feature when a lab calls for a specified mass of a substance.) CAUTION Never discard chemical or broken glassware into the wastepaper basket. This is an important safety precaution against fires, and it prevents personal injuries to anyone who empties the wastepaper basket. As a precaution against contamination, never pour unused chemicals back into their original bottles. PART 4 FILTRATION Apparatus Ring stand Stirring rod Ceramic center, wire gauze Materials Water Fine Sand Evaporating dish Matches Burner Wash Bottle Filter paper Iron ring Funnel Procedures 1. Sometimes liquids contain particle of insoluble solids, present either as impurities or as precipitate formed by the interaction of the chemicals used in an experiment. If the particles are insoluble and denser that water, they soon sink to the bottom. Most of the clear, supernatant (swimming above) liquid may be poured off without disturbing the precipitate. Such a method of separation is known as decantation. The soluble salts are in the supernatant liquid which has been decanted. 7 Figure 1.3 2. Fine particle, or particles that settle slowly, are often separated from a liquid by filtration. Support a funnel on a small ring on the ring stand as shown on Figure 1.3. If the ring is too large place a clay triangle on top of the ring to support the funnel Use a beaker to collect the filtrate. Adjust the funnel so that the stem of the funnel just touches the inside wall of the beaker. This will speed up the filtration process by decreasing the dropping, hence increases the continuous flow through capillary action due to the intermolecular force, hydrogen bonding. 3. Fold a circular piece of filter paper along its diameter, and then fold it again to form a quadrant. See Figure 1.4. Separate the folds of the filter, with three thicknesses on one side and one on the other; then place it into the funnel. The funnel should be wet before the paper is added. Use your plastic wash bottle to do this. Then wet the filter paper with a little water and press the edges firmly against the sides of the funnel so no air can get between the funnel and the filter paper while the liquid is being filtered. 4. EXCEPTION: A filter should not be wet with water when the liquid to be filtered does not mix with water. WHY? Figure 1.4 5. Dissolve between 2 and 3 grams of sodium chloride, and mix 2 to 3 grams of fine sand into a beaker containing about 50ml of distilled water. Then filter out the sand by pouring the mixture into the filter, observing the following suggestions: a. The filter paper should not extend above the edge of the funnel. It is better to use a filter disc that leaves about 1cm of the funnel exposed. b. Do not fill the filter. It must never overflow. The liquid must never go above the paper line. c. Try to establish a water column in the stem of the funnel thus excluding air bubbles, look at # 2, and then add the liquid just fast enough to keep the level about 1cm from the top of the filter paper. d. When a liquid is poured from a beaker or other container, it may adhere to the glass and run down the outside wall. This may be avoided by holding a glass stirring rod against the lip of the beaker, as shown in Figure 1.3. The liquid will run down the rod and drop off into the funnel without running down the side of the beaker. e. To remove the remaining sand from the beaker. Hole the beaker with its spout down towards the funnel and squirt your wash bottle up into the beaker to wash out the sand product. This is a useful skill. 6. The sand is retained on the filter paper. What PROPERTY of the sand enables it to be separate from the water by filtration? 8 7. What does the filtrate contain? 8. The salt may be recovered from the filtrate by pouring the filtrate into an evaporating dish and evaporating it nearly to dryness. See Figure 1.5 for the correct setup. 9. Turn back the flame as soon as the liquid begins to spatter. What PROPERTY of salt prevents it from being separated from the water by filtration? 10. At the end of this part of the experiment, all equipment you store in the lab drawer should be completely cool, clean, dry, and arranged in an orderly fashion for the next lab experiment. 11. Check to see that the valve on the gas jet is completely turned off. Make certain that filter papers and sand are thrown into the waste jar or container provided by your teacher and NOT down the sink. Wash your hand thoroughly before leaving the lab. Figure 1.5 PART 5 MEASURING LIQUIDS Figure 1-11 Apparatus Graduated Cylinder Buret Clamp and Buret Pipet Ring stand Beakers Materials Water CAUTION Safety goggles and apron must be worn whenever you measure chemicals. Never pour a liquid directly from its reagent bottle into the buret. You should first pour the liquid into a small beaker that is easy to handle. Then pour the liquid from the small beaker into the buret. This simple method will prevent unnecessary pillage. Never pour any unused liquid back into the reagent bottle. 9 Procedures 1. Burets, fitted with a stopcock, a pinch clamp, or a glass bead, are used for delivering any desired quantity of liquid up to the capacity of the buret. Any burets are graduated in tenths of milliliters, See Figures 1.6 . When using a buret, follow these steps: a) The buret has been clamped into positions on a ring stand. See Figure 1.6. Bring a small beaker with you to the buret. DO NOT move the buret or ring stand. b) Fill the buret with water and then draw off enough liquid to fill the tip below the stopcock and bring the level of the liquid down to the scale. Place a beaker at the bottom of the buret. The beaker serves to catch any liquid that will be drawn off. The height at which the liquid stands is them read accurately. Practice this procedure several times by pouring water into the buret and emptying it through the stopcock. Figure 1.6 Figure 1-7 c) Observe that the surface of the liquid in the buret is slightly curved. It is concave if it wets the glass, convex if it does not wet the glass. Such a curved surface is called a meniscus. If the liquid wets the glass, you read to the bottom of the meniscus as shown in Figure 1.7. You must read the mark at the bottom of the meniscus. Locate the bottom of the meniscus when reading the water level in the buret. d) When you have practiced a few times, empty your beaker. e) Measure and record the buret reading below. Remember to record your measurement to the correct precision. f) Open the stopcock and allow between 10ml and 14ml to dispense into your beaker. Close the stopcock. g) Read and record you final buret reading below. _________________________________ h) Subtract your measurements from e) and g) to obtain the actual amount of water dispensed. Show your work. Keep the water in your beaker for the next part of this procedure. ____________________________________________________ 10 2. Pipettes are made in many sizes and are used to deliver measured volumes of liquids. A pipet is fitted with a suction bulb used to withdraw air from the pipet while drawing up the liquid to be measured. See Figure 1-8. Always use the suction bulb – NEVER pipet by mouth. Use your thumb and forefinger to press on valve "A" and squeeze the bulb with other fingers to produce a vacuum for aspiration. Release valve "A" once the bulb is completely deflated. Insert the pipette into the water in your beaker. Press on valve "S". Suction will draw liquid up into the pipette. Continue pressing valve "S" until the all the liquid has been transferred to the pipette. Record the volume of water that is in your pipette. Make sure you record your measurement to the correct precision. Figure 1.8 bulb to be used with pipette ___________________________________ml Note: Press directly on the letter A, S, or E when opening valves. Applying pressure away from the center of these valves will damage them. 3. For approximate measurements of liquids, a graduated cylinder such as the one shown to the right is generally used. These cylinders are usually graduated in milliliters (ml). Such a graduated cylinder may read from 0 to 10ml, 0 to 25ml, 0 to 50ml or more, from bottom to top. It may also have a second row of graduations reading from top to bottom. Examine your cylinder for these markings. 4. Carefully hold the pipet so that the tip is inside you graduated cylinder. Gently press the E button on the bulb to dispense the water from the pipet into your graduated cylinder. 5. Read and record the amount of water that is in your graduated cylinder. 6. Compare the measurements you obtained using the three measuring devices. Typically, a graduated cylinder is used to measure liquids in a laboratory setting. Hypothesize at least one advantage and one disadvantage of using a graduated cylinder in the lab over a pipet or buret. 7. At the end of this part of the experiment, the equipment you store in your lab drawer should be clean, dry, and arranged in an orderly fashion for the next lab experiment. 11 PART 6 MEASURING TEMPERATURE AND HEAT Apparatus Calorimeter Hotplate Ceramic-centered wire gauze Thermometer Beaker Tongs Graduated Cylinder Beaker Materials Water Figure 1-9 Procedures 1. A thermometer is used to measure temperature and temperature changes. Examine your thermometer and the temperature range for the Celsius temperature scale. Compare the Celsius scale with the Fahrenheit scale as shown above. 2. Examine the calorimeter pictured in Figure 1-9. A calorimeter is an apparatus used in measurements involving heat and heat transfer. For approximate measurements, the simple calorimeter that is shown may be used. The small quantities of heat that may be transferred to or from the calorimeter may be disregarded because of the insulating characteristics of the metallic material of which it is composed. 3. Measure 50ml of tap water in a graduated cylinder, and pour it into the center of your calorimeter. Record the temperature of the water in Data Table I. Set the calorimeter and water aside for the moment. 4. Measure 50ml of tap water into a graduated cylinder, and pour it into a 250ml beaker. Warm the water to about 60oC by heating it on a hot plate. When heating water in a beaker, ALWAYS have your beaker tongs and heat resistant ceramic pad ready to use. The beaker becomes hot after heating. Use beaker tongs when handling the beaker of hot water. 12 CAUTION. 5. As soon as the temperature reaches around 60oC, take the beaker off the ring stand and place it on the heat resistant pad. Stir the liquid for 30 seconds then take the temperature. This ensures equal heating hence consistent temperature throughout the liquid. Record the temperature of the hot water in Data Table I. Then, using beaker tongs to hold the beaker immediately transfer the hot water to the calorimeter. Replace the lid, insert the thermometer, and stir the contents gently until a fixed temperature is reached,(temp stops changing) Record this final temperature in Data Table I. 6. At the end of this experiment, all equipment you store n the lab drawer should be completely cool, clean, dry, and arranged in an orderly fashion for the next lab experiment. Check to see that the valve on the gas jet is completely turned off. Remember to wash your hands before leaving the lab. DATA TABLE I Volume of cold water __________________________ ml Temperature of cold water ____________________ oC Volume of hot water _________________________ ml Temperature of hot water ____________________ Final temperature of mixture ___________________ oC oC You will need to know that the density of water is 1.00g/ml 7. Please turn to the back of your packet (page 15-16) and complete the post lab calculations for this section. Post Lab QUESTIONS Answer the following questions using complete sentences. Remember, all answers should be your own words. 1. Before doing an experiment, what should you read? 2. What immediate action should you take when the flame of your burner is burning inside the base of the barrel? 3. What is a common cause of fires in lab drawers? 13 4. Why are broken glassware, chemical, matches, etc. never thrown into a wastepaper basket? 5. Why should you never touch chemicals with your hands? 6. What precautions can you take against chemical contamination in reagent bottles? 7. Why chemicals and hot objects are never placed directly on a balance? 8. What is the rule about the size of the filter paper to be used with a funnel? 9. How can a liquid be transferred from a beaker to a funnel without spattering and without running down the outside wall of the beaker? 10. In what condition should all lab equipment be stored at the end of an experiment? What else should be checked? 11. How should a thermometer be placed in a liquid for an accurate temperature reading? __________________________________________________________ 14 12. How is the filter funnel placed in a beaker to ensure faster filtration? ___________________________________________________________ 13. As soon as you enter the lab, what standard safety equipment should you put on immediately? 14. Before you light a burner, what safety precautions should always be followed? 15. What type of flame is preferred for laboratory work and why? 16. What is never used to measure a precise volume of liquid? ________________________________________________________________ 17. List three instruments used in the laboratory that are used for measuring small quantities of liquids. What precaution should be taken when filling a buret with a liquid? Calculations from part 6 Complete the calculations below. Make sure that you show all of your work. Circle your answer. 18. Calculate the temperature change of the cold water. This is calculated by subtracting the initial temperature of the cold water (before mixing) from the final temperature (after mixing). 19. Calculate the temperature change of the hot water. This is calculated by subtracting the final temperature (after mixing) from the initial temperature of the hot water(before mixing). 20. The quantity of heat, Q that is gained and lost may be calculated from the following equation: Q( in calories) = temperature change (oC) x mass (g) x 1.0cal/g oC Recalling that energy in any form cannot be created or destroyed, look at your numbers to see if the lab data can support this law of conservation?_____________________________________________________________ __________________________________________________________________________________ 15 21. Using the equation provided, calculate Q for the heat gained by the 50ml of cold water. Assume that 50ml of water has a mass of 50g. Q( in calories) = temperature change (oC) x mass (g) x 1.0cal/g 22. Using this equation, calculate Q for the heat lost by the 50ml of hot water. Assume that 50ml of water has a mass of 50g. 23. How should these two quantities of heat compare to each other? Recalling that energy... 24. Provide an explanation as to why your data does not show conservation of energy. _______________________________________________________________________________ __________________________________________________________________________________ Please note, if you have not already completed the proceeding post lab questions 1-17 do so now. SAFETY CHECK - Identify the following safety symbols: 16 CHEMICAL APPARATUS Identify each piece of apparatus. Place your answers in the cell with the picture in it. 17 TRUE or FALSE Read the following statements and indicate whether they are true or false. Place your answer in the space next to the statement. 1. Never work alone in the laboratory. 2. Never lay the stopper of a reagent bottle on the lab table. 3. At the end of an experiment, save all excess chemical and pour them back into their stock bottles. 4. The quickest and safest way to heat a material is a test tube is by concentrating the flame on the bottom of the test tube. 5. Use care in selecting glassware for high temperature heating. Glassware should be Pyrex or a similar heat-treated type. 6. A mortar and pestle should be used for grinding only one substance at a time. 7. Safety goggles protect your eyes from particle and chemical injuries. I do not need to make my teacher aware of the fact that I wear contact lenses. 8. Never use the wastepaper basket for disposal of chemicals unless told to do so by your teacher. 9. First aid kits may be used by anyone to give emergency treatment after and accident. 18 Honors Chemistry Name______________________________ Lab 1: LABORATORY PROCEDURES – Lab Safety and Techniques – 80 points _____/1 1. Name Period and dates(1 pt) _____/4 2. Part I completed by the student in the lab within one week of the lab day assigned (4 points) _____/4 3. Part 2 & 3 Lab completed by the student in the lab within one week of the lab day assigned (4) _____/4 4. Part 4 Lab completed by the student in the lab within one week of the lab day assigned (4) _____/4 5. Part 5 Lab completed by the student in the lab within one week of the lab day assigned (4) _____/19 6. All questions in packet completed(19 pts) _____/5 7. Calculations in questions 1-5 show work and include unit label. ( 5 pts) _____/10 _8. Five of the 17 post lab questions (randomly selected by the teacher) are accurate (10 pts) _____/10 9. Safety check ( 10 pts) _____/10 10. Chemical apparatus (9 pts _____/9 11. True/False (9 pts) _____/0 12. All questions answered in complete sentences (1.5 pts extra credit) _____/80 TOTAL 19