Exam 3
advertisement

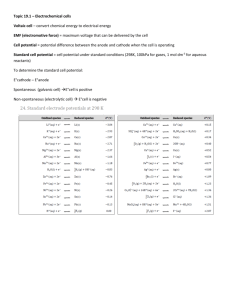
Chemistry 116: General Chemistry Syracuse University Project Advance Exam #3, Spring 2006 Name Date The last pages of this examination are reference tables. [Gas constant = 0.008314 kJ/mol K; 1 faraday = 96500 coul/ mol e- ] 1) If H = -150 kJ/mol for a given process, then it A. B. C. D. E. needs a catalyst does not need a catalyst occurs slowly is endothermic is exothermic 2) For a spontaneous process, which one of the following is always positive? A. G B. H C. Eo D. S E. None of these 3) For the reaction CH4(g) + N2(g) + 164 kJ HCN(g) + NH3(g) at 25°C and 1 atm pressure, G 159kJ. Calculate S at 25°C. A. B. C. D. E. 0.2 J/K 200 J/K 0.01677 J/K 16.77 J/K None of these 4) Which of the following processes has the largest positive value for S˚? A. B. C. D. E. Ba(OH)2(s) + CO2(g) BaCO3(s) + H2O(l) N2(g) + H2(g) 2NH3(g) 2SO3(g) 2SO2(g) + O2(g) AgNO3(aq) + HCl(aq) AgCl(s) + HNO3(aq) 2Hg(l) + O2(g) 2HgO(s) CHE 116 1 Spring 2006 Exam #3 5) Given that G˚ = 91.7 kJ/mol for the reaction, Ag+(aq) + I-(aq) AgI(s), calculate the value of Ksp for AgI at 25˚C. A. B. C. D. E. 1.6 x 10-29 8.3 x 10-17 4.5 x 10-14 9.1 x 10-9 6.6 x 10-7 6) If H for a process is -64 kJ/mol and S is -154 J/mol K, the process will be spontaneous A. B. C. D. E. above 416 K below 416 K above 3570 K at all temperatures at no temperatures 7) Which of the following is true when one mole of CO2(g) changes to dry ice? 1. The entropy decreases. 2. The entropy increases. 3. The enthalpy decreases. 4. The enthalpy increases. A. B. C. D. E. 1 only 2 only 1 and 4 only 2 and 3 only 1 and 3 only 8) Calculate the Go for the following reaction at 500 K. Cu (s) + H2O (g) CuS (s) + H2 (g) Hof (kJ/mole) 0 -241.8 -155.2 0 Cu (s) H2O (g) CuO (s) H2 (g) A. B. C. D. E. CHE 116 So (J/K) 33.3 188.7 43.5 130.6 +110.6 kJ -86.6 kJ +23.9 kJ -62.6 kJ +301 kJ 2 Spring 2006 Exam #3 9) For the reaction: 3C (s) + 4H2 (g) C3H8 So = -29 J/mole *K Ho = -103.8 kJ/mole Calculate the equilibrium constant at 25oC for the reaction above. A. B. C. D. E. 1.04 0.962 2.09 x 10-17 4.79 x 1016 2.1 x 1032 10) Calculate the voltage of a cell in which the following reaction occurs: Zn (s) + 2H+ (aq, 0.001 M) Zn+2 (aq, 1M) + H2 (g, 1 atm) Zn+2 + 2e- Zn The standard reduction potential for zinc is: A. B. C. D. E. Eo = -0.763 v +0.73 v +0.41 v +0.58 v +0.70 v -0.41 v 11) Electrolysis refers to an electrochemical reaction that is A. B. C. D. E. exothermic. spontaneous. nonspontaneous, but forced to proceed. voltaic. oxidation-reduction. 12) What is the reducing agent in the following reaction? PbO2(s) + Pb(s) + 4H+(aq) + 2SO42-(aq) 2PbSO4(s) + 2H2O(l) A. B. C. D. E. PbO2 Pb H+ SO42PbSO4 CHE 116 3 Spring 2006 Exam #3 13) The flow of electrons through the external circuit attached to a voltaic cell is A. from the anode to the cathode. B. from the cathode to the anode. C. only predictable once one knows what the half-reactions are. 14) Which of the following reactions is a redox reaction? I. K2CrO4 + BaCl2 BaCrO4 + 2KCl II. Pb22+ + 2Br- PbBr III. Cu + S CuS A. B. C. D. E. only I only II only III I and II all three (I, II and III) 15) Use the standard reduction potentials: Cu2+ Cu+ +0.16 V I2 I+0.54 V Calculate the value of E° (in V) for a cell in which the overall reaction is 2Cu+ + I2 2Cu2+ + 2IA. B. C. D. E. 0.38 V –0.38 V 0.68 V –0.68V 0.83 V 16) Of the following, which is the strongest oxidizing agent? A. B. C. D. E. Ni2+ Li+ Fe2+ F2 Al 17) What process occurs at an anode? A. B. C. D. oxidation reduction oxidation and/or reduction neither oxidation nor reduction CHE 116 4 Spring 2006 Exam #3 18) When the basic solution redox equation, BrO- + Fe(OH)2 Br- + Fe(OH)3, is properly balanced, the coefficients of the reactants and products shown are, in order, A. B. C. D. E. 1, 1, 1, 1 1, 2, 1, 2 2, 1, 2, 1 1, 3, 1, 3 2, 5, 2, 5 19) If a piece of tin and a piece of copper were submerged in a solution containing SnSO4 and CuCl2, what would happen? Cu+2(aq) + 2e- Cu(s) E° = 0.337 V Sn+2(aq) + 2e- Sn(s) E° = -0.136 V A. B. C. D. E. The tin electrode would gain weight and the copper electrode lose weight. The tin electrode would gain weight and the copper electrode gain weight. The tin electrode would lose weight and the copper electrode lose weight. The tin electrode would lose weight and the copper electrode gain weight. Nothing would happen. 20) Which of the following is(are) true about an electrochemical reaction that is spontaneous in its standard state? A. B. C. D. E. The standard electromotive force is positive. The standard free energy decreases. The entropy of the universe increases. All of the above. None of the above. 21) The two electrodes Cr(s)/Cr3+(aq) and Sn(s)/Sn2+(aq) are combined to afford a spontaneous electrochemical reaction. The standard reduction potentials in V for Cr3+(aq) and Sn2+(aq) are –0.74 and –0.14, respectively. E° in V for this cell is A. B. C. D. E. +0.88 –0.88 +0.60 –0.60 +2.50 CHE 116 5 Spring 2006 Exam #3 22) What is the half-reaction occurring at the cathode in the voltaic reaction below? 3MnO4-(aq) + 24H+(aq) + 5Fe(s) 3Mn2+(aq) + 5Fe3+(aq) + 12H2O(l) A. B. C. D. E. MnO4-(aq) + 8H+(aq) + 5e- Mn2+(aq) + 4H2O(l) 2MnO4-(aq) + 12H+(aq) + 6e- 2Mn2+(aq) + 3H2O(l) Fe(s) Fe3+(aq) + 3eFe(s) Fe2+(aq) + 2eFe2+(aq) Fe3+(aq) + e- 23) Lithium is produced by the electrolysis of lithium chloride as follows: 2 LiCl (l) 2 Li (l) + Cl2 (g) During passage of a certain current for 45 minutes, 169 grams of lithium were produced. How many liters of Cl2 gas (measured at STP) were produced? A. B. C. D. E. (169)(22.4) / (70.9) (45)(60)(169) / (2)(96500) (169)(22.4) / (6.94)(2) (45)(60)(22.4) / (96500) (169)(70.9) / (6.94) EXTRA CREDIT: Consider the cell: Zn(s) | Zn+2 (0.10 M) || H+ (aq) | H2 (g) (1 atm) This cell can be used to provide a measure of the pH in the cathode compartment. What is the pH in the cathode compartment if the cell emf is measured to be 0.60 v when [Zn+2]=0.10 M and P H2 = 1 atm? (recall pH = -log[H+] ) SHOW ALL WORK CHE 116 6 Spring 2006 Exam #3