Midterm Exam Review
advertisement

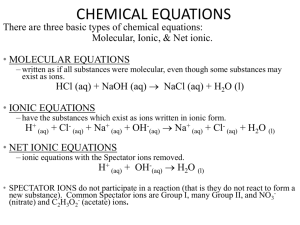
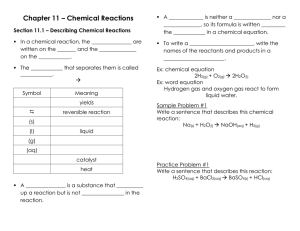
Midterm Exam Review Chapters 1-9 1. What is the difference between and theory, a law, and a hypothesis? “When I drop a ball it will fall” is an example of which? 2. List the three steps of the scientific method. 3. In an experiment to determine if the height from which you drop a watermelon determines how far it splatters when it hits the ground, identify the independent, dependent variables, control and 3 constants. 4. How many significant digits are in the following: a. 1.004 b. 0.0003010 5. Calculate the following. Round answers to the correct significant digits a. 1.34 x 2.0 b. 2.9010 + 387.45 6. Write the following in scientific notation. a. 0.000381 b. 38100000 7. Convert the following: a. 7.990daL to cL b. 34.9nm to mm 8. Convert 34.9°C to °F 9. Convert 893°C to K 10. Would the burning of magnesium be a physical or chemical change? Explain. 11. Draw a diagram of a. a solid compound b. a gaseous mixture of an element and a compound 12. Magnesium oxide, air, sugar, gold. Which of these are pure substances? 13. What is the difference between mass and volume? 14. An object has a mass of 45.69g and when you immerse it in water, the volume changes from 67.89mL to 120.44mL. a. What is the density of the object? b. Will this object float in ethanol? (density=0.94g/mL) explain. 15. Is density a physical or chemical property? What about flash point (the temperature at which something begins to burn)? 16. Would the following be elements, compounds, heterogeneous or homogeneous mixtures? a. Salt water b. salad c. polyester 17. How do natural laws require the intervention of a creator at the beginning of the universe? 18. Compare and contrast Young Earth Creation and the Big Bang. 51 19. How many protons, neutrons and electrons are in 23V2- 20. That element would have a mass number of 56 with 32 neutrons? 21. What contribution was made by the following people? a. Dmitri Mendeleev b. John Dalton c. J.J. Thomson d. Ernest Rutherford 22. Give 2-3 identifying properties of each of the following groups of elements. (location on the table, reactivity, metal/nonmetal, other defining features) a. Halogens b. Alkali Metals c. Alkaline Earth Metals d. Noble gases e. Transition Metals 23. What is the charge on a calcium ion? An oxygen ion? A nitrogen ion? A cesium ion? 24. Would FeCl3 or N2O be an ionic compound? Explain. 25. Would the following be soluble or insoluble? a. Fe(NO3)3 b. CaS c. Na2CO3 d. Mg(OH)2 26. Name the following compounds a. K2SO4 b. CaS c. HI i. NH4Cl j. NO2 27. Write the formula a. Potassium Acetate d. Fe(ClO4)3 b. Cobalt (II) Hydroxide e. PdO c. Tin (II) Nitride f. HIO3 d. Nitric acid g. SrF2 e. Carbon tetrafluoride h. H2CO3 f. Calcium Phosphide k. Acetic acid g. Hydrochloric acid l. Ammonia h. Magnesium hydroxide m. Rubidium Iodide i. Molybdenum (III) Phosphite n. Sulfur trioxide j. Xenon hexafluoride Identify the following symbols 28. (g) 29. (aq) 30. → 31. Write the balanced chemical equation and identify the type of reaction: Sodium metal is added to water and aqueous sodium hydroxide and hydrogen gas are produced. 32. Complete and/or balance the reaction, then identify the type(s) of reaction: a. C2H5OH + O2 → CO2 + H2O b. Cl2 + K → KCl c. silver nitrate + hydrochloric acid → rewrite the formula and complete d. HNO2 + Ca(OH)2 → e. Ammonium nitrate → nitrogen + oxygen + water vapor 33. How many moles of Copper (II) nitrate are in 65.99g? 34. How many grams of dinitrogen tetroxide are in 5.66x1022 molecules? 35. What is the molar mass of sodium sulfite? 36. What is the percent composition of ammonium sulfide? 37. What is the percent composition of CaCO3? 38. Find the empirical formula of a compound that is 39.3 % carbon, 8.3% hydrogen, and 52.4% oxygen by mass. 39. Analysis of ibuprofen shows that it contains 75.7% carbon, 8.8% hydrogen, and 15.5% oxygen. The molar mass of ibuprofen is 210g/mol. Determine the empirical and molecular formula of ibuprofen. 40. Which of the following would be insoluble in water? Why? a. Ca(NO3)2, Fe2S3, KOH, BaCO3 41. Balance, then write the total and net ionic equation: a. K2CO3(aq) + CaCl2(aq) → KCl(aq) + CaCO3(s) i. Total ionic equation ii. Net ionic equation 42. Balance, then write total and net ionic equations a. NaOH(aq) + H2SO4(aq)→Na2SO4(aq) + H2O i. Total ionic equation ii. Net ionic equation 43. Use the reaction: 2LiOH(s) +CO2(g)→Li2CO3(s) + H2O(l) a. How many grams of carbon dioxide can be removed by 5.5mol LiOH? b. How many grams of H2O could be made when 3.28g of CO2 react? 44. Use the equation: 2NaN3(s)→2Na(s) + 3N2 a. How many grams of Na can be made form 31.1g of NaN3? b. How many moles of N2 can be formed from 2.7mol NaN3? 45. 2AgNO3 + NiCl2 → 2AgCl + Ni(NO3)2 How many grams of silver nitrate are required to react with 46.99g of nickel (II) chloride?