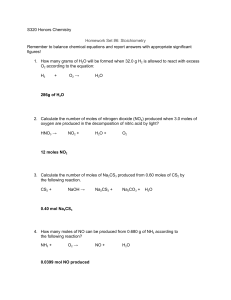
Day 2 review
advertisement

PreAP Final Exam review Chapters/ Topics for Day 2 Final Electron configuration Periodic table trends Moles EM spectrum Bonding Nomenclature 1. How many electrons occupy the following energy levels: a. n=1 b. n=2 c. n=3 d. n=4 2. Electrons are located in __________________ surrounding the nucleus. 3. Write the Electron Configuration, Noble Gas Notation, and Orbital Notation for: a. Mg b. Br c. Ni 4. Define/understand: Pauli’s Exclusion principle, Hund’s Rule, and the Aufbau principle 5. What is the relationship between wavelength/frequency/energy? Which ones are directly related, which ones are indirectly related? 6. Which types of waves & colors of the visible light spectrum have the highest & lowest: energy, frequency, wavelength 7. What are the units for energy, frequency, and wavelength? 8. Calculate the following: a. If you have a frequency of 3.55 x 1017 Hz determine the energy. b. If you have a wavelength of 5.0 x 10-7 m, what is the frequency? 9. Define and identify the Periodic Trends: Ionization Energy, Electronegativity, Ionic Radius, Atomic Radius 10. Which element has the highest Electronegativity? 11. Which element has the biggest Atomic Radius? 12. Define: ionic bond, covalent bond (molecular compound), and metallic bond. 12. Fill out the table: Symbol/Formula compound/ Element Ionic, covalent, or metallic transfer of esharing of esea of e- Cu H2O NaCl MgSO4 C6H12O6 13. Define Lewis structure. What is used to draw them. 14. Draw a Lewis structure showing the bonds for: HBr, CCl4, H2O 15. Show how the following ions combine. Show the transfer of electrons along with the appropriate charges. Draw the final formula. a. Mg and Cl b. K and N c. Al and O 16. When drawing Lewis structures (dot diagrams) how many does every element need? What is the exception and how many does is need? 17. How many valence electrons do groups number 1,2, 13, 14, 15, 16, 17, 18 have? What is group 18 called and why are they special? 18. What is the oxidation numbers (charges for each of the above groups in #17? 19. Name the following compounds: a. CuCl2 b. NaCl c. FeBr3 d. H2O e. (NH4)2CO3 f. N2Cl g. Fe(OH)2 h. CCl4 i. Ca(C2H3O2)2 j. Na2SO4 20. Write the formulas for the following: a. iron (II) nitrate b. aluminum oxide c. potassium iodide d. sodium nitrate e. copper (I) nitrate f. iron (III) oxide g. iron (III) hydroxide h. carbon monoxide i. zinc nitrate j. dihydrogen pentasulfide k. lead (II) sulfate l. potassium chromate 21. Find the molar masses of the following: a. b. c. Ca(OH)2 Na3PO4 (NH4)2CO3 22. Calculate the following: a. How many moles are in 25.0 grams of water, H2O? b. How many grams are in 4.50 moles of Lithium Oxide, Li2O? c. How many molecules are in 23.0 moles of oxygen, O2? d. How many molecules are in 25.0 grams of ammonia, NH3? e. How many atoms of Oxygen are in 60 g of baking soda (NaHCO3)? Remember baking soda is a formula unit!!! This is a three step problem!