the Chemistry of Life
advertisement

the Chemistry of Life
Elements in the Human Body
Bonding
The Carbon Atom
Carbohydrates
Lipids
Proteins
Nucleic Acids
ATP
Handout: Chemistry of Life Assignment 1 – complete as directed
1. Elements in the Human Body
Notes:
along with oxygen (O) and hydrogen (H) – which combine to form water
(H2O) – carbon (C) is the most important element to life
organic chemistry = the study of carbon compounds
cells are 70 – 95% water, the rest is carbon-based compounds
enzymes, proteins, carbohydrates, lipids, DNA, and ATP (energy storage
molecules) are all made of carbon atoms covalently bonded to other elements
Glencoe textbook – see table 6.1, p. 146
Activity: Write out the element name, symbol, and % by mass of all the
elements that occur in > trace amounts in the human body.
2. Bonding
Notes:
covalent bonds share electrons (e-)
sharing electrons creates a force that holds the atoms tightly together
energy (nrg) can be released when covalent bonds are broken
nrg is required to form bonds
Two simplified models of a helium (He) atom.
The helium nucleus has 2 neutrons and two protons. Two electrons move rapidly
around the nucleus in a very large orbit (diagram is not to scale).
The protons have a positive charge; the neutrons have no charge and the
electrons have a negative charge.
- A single covalent bond consists of a pair of shared electrons.
- The number of electrons required to complete an atom’s valence shell generally
determines how many bonds that atom will form.
3. The Carbon Atom
Notes:
the valence (# of outer shell e- available to form bonds) of carbon allows it to
bond with many diff types + numbers of atoms
eg. methane
4 single bonds
(draw)
eg. carbon dioxide CO2
2 double bonds
(draw)
eg. ethyne
(acetylene)
1 triple, 2 single bond (draw)
CH4
C2H2
Methane
3. a) Organic Compounds
Notes:
Organic compounds:
can be broken down to release nrg
can be linked together to form macromolecules
are carbon compounds
occur naturally only in living things or their products
contain hydrogen and oxygen
may contain nitrogen (N), phosphorus (P), sulfur (S), iron (Fe) , calcium (Ca),
sodium (Na), chlorine (Cl), magnesium (Mg), or potasium (K)
Valences of the major organic molecules
3. b) Inorganic Compounds
Notes:
Inorganic compounds:
usually do not contain carbon
CO2 and CaCO3 are exceptions (carbon dioxide and calcium carbonate are not
organic)
living things do contain some inorganic compounds
eg. water, iron (Fe in hemoglobin), inorganic salts, carbon dioxide,
inorganic acids and bases
Note: if a compound is NOT organic, it IS inorganic
3. c) Structure of Carbon Compounds
Notes:
The structure of carbon compounds:
is due to carbon’s bonding characteristics
allows for the formation of 4 covalent bonds
see handout for the following
Name
Chemical Formula
Ethane
C2H6
Ethene
C2H4
Ethyne
C2H2
Water
H2O
Benzene
(ring)
C6H6
Glucose (ring)
C6H12O6
Glucose (linear)
C6H12O6
Structural Formula
(draw)
(draw)
(draw)
(draw) not linear
(draw) alt sngl/dble
(draw)
(draw)
Fructose
C2 instead of C1
Galactose
C6H12O6
C6H12O6
(draw) dbond is on
(draw)
Handout: Identifying Organic Compounds
(ie. organic vs. inorganic molecules)
Performance Assessment marks =
½ x 26 lines = 13 marks
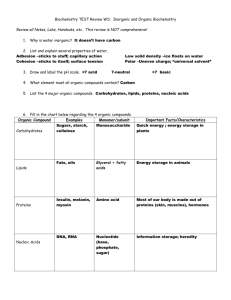
4. Carbohydrates
a) Role of Carbs in Living Systems
plants (using PS) make carbohydrates
carbs are the primary source of nrg in most living systems
Brainstorm: In your table groups, think of as many common foods as you can
that you know are high in carbs.
b) Monosaccharides, Disaccharides, and Polysaccharides
the above translate as “single sugars”, “double sugars”, and “many sugars”
carbohydrates are made up of the elements C, H, O
monosaccharides:
are carbs made of only one sugar
may have the same chemical formula, but be different compounds due to
their diff structure
eg. C6H12O6 = glucose, fructose, and galactose
CD: 5C Carbohydrates
disaccharides:
are formed when 2 simple sugars join together
eg. glucose + glucose = maltose
eg2. glucose + fructose = sucrose
Dehydration Synthesis
sugar molecules are bonded together in a process called dehydration
”removing water” synthesis “to make”
{example with 2 glucose molecules to form maltose}
Hydrolysis (“water”, “to cut” or “break”)
the breaking of bonds in a compound by the addition of a water molecule
{example of maltose being broken into 2 glucose molecules}
Polysaccharides
organisms store excess sugar in the form of polysaccharides
in plants: as starch in seeds, roots, and stems
in animals: as glycogen in the liver (also some in the muscles, but this is
short-term storage and must be constantly replaced)
plants use polysaccharides for structural strength in the form of cellulose
some insects also use polysaccharides for strength in shells in the form of
chitin
Chitin is in the exoskeleton of arthropods (shedding cicada), and can be used as
surgical thread that will breakdown after the wound heals.
5. Lipids
b) Structure of fats, oils, and waxes
fats and oils are chemically similar; are hydrophobic; dissolve fat-soluble
vitamins
fats are solid at room temperature (saturated); oils are liquid (unsaturated)
1 molecule of fat or oil is produced by the dehydration synthesis of 3 fatty
acids to 1 glycerol molecule (removal of 3 x H2O)
fatty acids have 2 parts: a chain of C atoms with bonded H atoms; plus a
carboxyl group (-C=O)
glycerol is a simple 3-C chain with an –OH group on each C
waxes are lipids formed by the combination of fatty acids with compounds
similar to glycerol
c) Saturated and Unsaturated Fats
if all the C-H bonds in the fatty acid are single, then the lipid is saturated (fat)
if one or more pairs of carbon atoms are joined by double or triple bonds,
then the lipid is unsaturated (oil)
polyunsaturated is the term for >1 dble or triple bond in the oil
kinks in the structure caused by dble and triple bonds means the fatty acid
tails cannot pack together closely and are therefore not solid
unsaturated fats can be hydrogenated to become solid eg. peanut butter
draw and label a lipid molecule
d) Cholesterol
is a type of lipid called a steroid
have a characteristic 4 ring structure
used to make sex hormones in the h.b.; eg. estrogen and testosterone
is an essential compound giving structure to cell membranes
also appears to play a role in the buildup of deposits that harden and narrow
arteries (may lead to heart disease)
consumption of saturated fats (butter; in meats) is thought to increase the
amount of cholesterol produced by the body; while unsaturated and
polyunsaturated fats (found in plant products) are thought to decrease blood
cholesterol levels
(complete concept map for carbs and lipids)
6. Proteins
Uses in the body:
structural parts in cells and body tissues eg. cartilage, bones, muscles
in hormones – the chemical messengers that regulate body functions in
animals and plants
in antibodies – substances that protect against disease
in enzymes – to allow complex chemical reactions to take place
names usually end in -in
Structure:
contain nitrogen (N) as well as C,H, and O
some contain sulfur (S) and phosphorus (P)
building blocks are called amino acids
amino acids:
are the structural units
there are 20 diff. amino acids
have a central C bonded to:
1 carboxyl group (-COOH)
1 amino group (-NH2)
1 H
1 variable side chain (-R); 20 diff. ones
eg. glycine has an H that is its side chain
eg. alanine has a –CH3 group
Bonding
the bond that forms by dehydration synthesis b/w a.a. is called a peptide bond
the molecules are called amino acids dipeptides polypeptides
proteins have at least 3, and usually 4 levels of structure (as explained in
class)
the primary level is the sequence that the a.a. are linked
the secondary level is the twisting and folding:
eg. coils, helixes, pleated sheets
the tertiary level of structure is the 3-D shape of the globule
the quaternary level is when more than 1 (usually 4) polypeptide subunits
combine to form a functional protein
Enzymes
are the proteins responsible for most of the chemical reactions in living cells
usually speed up reactions (rxns) (organic catalysts)
work on substances referred to as the substrate in the rxn
usually end in -ase
eg. maltase or sucrase work on sugars
eg. proteases break down proteins
lipase breaks down lipids
Substrates:
fit on the enzyme at a location called the “active site”
may cause 2 substrates to join:
each substrate has its own active site
enzyme’s shape changes to bring the substrates close enough for a bond to
form {by d.h.s!}
Lock and Key Model
explains how the enzyme and substrate bind
active site is the same shape as the substrate – no other shape will fit
Induced–Fit Model
enzyme’s shape changes when the substrate enters the active site – the
enzyme “grasps” the substrate
Factors Affecting Enzyme Activity
Amount of substrate
small amounts of an enzyme can cause the rxn of large amounts of
substrate
Temperature
enzymes enable cell reactions to take place at normal temperatures (don’t
need high T – normal b.t. is usually optimal)
pH
each enzyme works best at a certain pH (stomach enzymes work best at
low pH; intestinal enzymes best at higher pH)
Concentration relates to rate of reaction
the concentration of substrate and enzyme determines the rate that the
rxn will take place
Co-enzymes (cofactors)
coenzymes are organic substances other than enzymes
some are built into the structure of the enzyme and have to bind to the
enzyme in order for it to be functional
eg. vitamins
7. Nucleic Acids
are compounds that are made of nucleotides that contain a phosphate group,
a sugar, and a nitrogenous base
may be DNA (deoxyribonucleic acid) or RNA (ribonucleic acid)
both were first found in the cell nucleus
DNA
has the sugar deoxyribose and is double stranded and forms a double helix
is the heredity information that passes from generation to generation
RNA has the sugar ribose and exists as a single strand
is used in protein synthesis
Nitrogenous bases
in DNA are adenine (A), thymine (T), guanine (G), and cytosine (C)
bonding in DNA is always A-T and C-G
in RNA thymine is replaced by uracil, so the bonding is A-U (and C-G)
Make a simple table comparing the characteristics of DNA and RNA
8. ATP
THE HIGH energy compound
adenosine triphosphate
made of adenine, a ribose sugar, plus 3 phosphates
produced when glucose is broken down in cellular respiration
nd and 3rd P
the highest energy is in the bond b/w the 2
when the high energy bond is broken, the energy is released
this is the most common way that energy is transferred in living systems
the energy from a single glucose molecule can form 36 molecules of ATP from
ADP and Pi
Complete the handout with Part D. Carbon Compounds and Part E. Lipids,
Proteins, & Nucleic Acids
Complete the Chapter 6 Assessment (pages 171 – 173):
- Understanding Main Ideas #1, 7, 9 – 11, 13-18
- Applying Main Ideas #22 and 23
- Thinking Critically #27
- Assessing Knowledge & Skills 1 – 3
*** Answer all questions with complete sentences (including the m/c or this will
be useless to you when you are studying for tests or the exam! ***