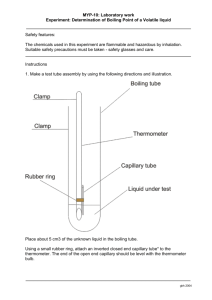
Worksheet for Video #5 - Safely Synthesizing Esters
advertisement

Worksheet for Video #5 - Safely Synthesizing Esters Complete this page after viewing the video and performing the investigation. 1. Since most organic compounds are combustible, the risk of unintentional combustion is always present when using these compounds. What are two possible energy sources that could ignite a volatile organic substance? How can the risk of these sources be minimized? 2. Identify two pieces of safety equipment that could be used to put out an unintentional fire in this investigation. 3. How can the risk involved with handling concentrated acids be minimized? 4. (a) What guideline(s) should be used when selecting the temperature of the water bath used in this investigation? (b) What document could you consult to find this information? 5. Why is clamping the test tube containing the ester synthesis mixture in the water bath not recommended? 6. What is the proper technique of smelling a gas in an investigation? (See page 31 in Safe ON Science for further details). 7. How would you manage the distribution of materials to your students during the ester synthesis activity to promote safety? 8. What are the three important safety considerations that you would discuss with your students prior to conducting this investigation? 9. What would you do if one of the student reaction mixtures ignited? Worksheet for Video #5 - Safely Synthesizing Esters Complete this page after viewing the video and performing the investigation. 1. Since most organic compounds are combustible, the risk of unintentional combustion is always present when using these compounds. What are two possible energy sources that could ignite a volatile organic substance? Open flames and heat sources. How can the risk of these sources be minimized? A water bath is the preferred heat source when warming any flammable liquid. Heat sources with open flames should not be used because the flame could ignite the combustible vapour. To prevent the buildup of excess vapours during investigations involving the heating of organic compounds the water bath should be kept several degrees below the boiling point of the compound and the lab well ventilated. 2. Identify two pieces of safety equipment that could be used to put out an unintentional fire in this investigation. Fire extinguisher and sand bucket. 3. How can the risk involved with handling concentrated acids be minimized? Stock bottles of concentrated acids should never be brought into the classroom. All decanting, dilution and/or preparation of concentrated acids should be done in the prep room fume hood. Small quantities of chemicals should be used so that the volume of concentrated sulfuric acid required can be kept to a minimum. The teacher should review the appropriate handling procedures for concentrated acids with students prior to the investigation and plan to dispense the concentrated acid. 4. (a) What guideline(s) should be used when selecting the temperature of the water bath used in this investigation? Methanol is a flammable and volatile liquid. To prevent the build up of excess vapours during ester synthesis the water bath should be kept several degrees below the boiling point of the alcohol (boiling point of methanol is 64.7°C) being used. (b) What document could you consult to find this information? The MSDS for each reactant and product should be referenced during the planning stages of the investigation and assessed for safety during use. 5. Why is clamping the test tube containing the ester synthesis mixture in the water bath not recommended? A test tube holder is used instead of clamping the test tube in place so that the reactants can be quickly removed from the water bath if there are any signs of boiling. Also, the use of a test tube holder provides more flexibility in holding the test tube in the water bath while ensuring it is pointed away from all other persons in the lab. 6. What is the proper technique of smelling a gas in an investigation? In scientific investigations the proper way of smelling a gas is to use the technique of wafting to avoid inhaling the gas. (See page 31 in Safe ON Science for further details). 7. How would you manage the distribution of materials to your students during the ester synthesis activity to promote safety? Dropper bottles prepared for each group ensure that no student has large quantities of any particular reagent. Have sign in and sign out sheet for equipment Make only small graduated cylinders or 1 mL pipettes available to your students to discourage the dispensing of more reactants than is called for in the procedure. Teachers should plan to dispense the concentrated sulfuric acid catalyst. 8. What are the three important safety considerations that you would discuss with your students prior to conducting this investigation? Do not bring lighters or matches into the classroom during this activity as there will be more volatile fumes than usual in the air Watch your reaction carefully while it is in the water bath and pull out the test tube at the first sign of boiling Do not exceed the recommended temperature of the water bath (review of pertinent information from MSDS sheets with students) 9. What would you do if one of the student reaction mixtures ignited? If the vapours in the test tube were to ignite the easiest solution may be to dump the contents of the test tube into the water bath, diluting the reagents and quenching the flame. In the case of a larger fire dumping the bucket of sand onto the surface is likely the next best step. The fire extinguisher can be used for larger fires or those that are not on flat, horizontal surfaces.