IB ChemistryScheme of Work
advertisement

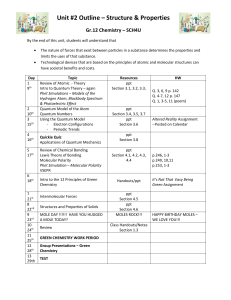
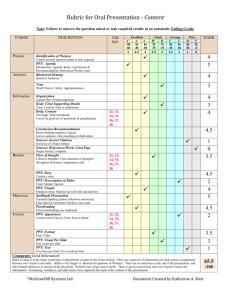
IB ChemistryScheme of Work Topic Lessons (1 hour blocks) 1: The mole concept and Avagadro’s Constant QuantitativeChemistry (12.5 hours) The mole concept and Avagadro’s Constant Formulas Formulas Formulas Chemical equations Mass and gaseous volume relationships in chemical reactions (+ 0.5 hours) Mass and gaseous volume relationships in chemical reactions Mass and gaseous volume relationships in chemical reactions Mass and gaseous volume relationships in chemical reactions Solutions Solutions Resources The mole ppt, The mole calculations worksheet 1 The mole ppt, mole calculations worksheet 2 Empirical formula ppt, Chemical names and formulas sheet, mole ppt 2 and mole handout Balancing act sheet, Chemical formula worksheet 2, Mg and crucible practical Worksheet formulas sheet, formula sheet Chemical equations ppt, Writing chemical equations info sheet, You tube clip, Calculating relative atomic mass ppt, Isotopes ppt, You tube video, Gases ppt, gas stoichiometry practice sheet Tough gas laws worksheet Solutions and titrations ppt, solutions worksheet Titration lab ppt, Homeworks The mole hwk sheet Chemical formulas and homework sheet Average atomic mass homework sheet Gas law question sheet Solubility homework 2: Atomic Structure (4 hours) The atom The Mass Spectrometer 3: Periodicity (6 hours) 4: Bonding (12.5 hours) 5: Energetics (8 hours) Electron arrangement Electron arrangement The Periodic Table Physical properties Physical properties Chemicalproperties Chemical properties Chemical properties Ionic bonding Ionic bonding Covalent bonding Covalent bonding Covalent bonding Covalent bonding Covalent bonding Covalent bonding Intermolecular forces Intermolecular forces Metallic bonding (0.5 hours) Physical properties Physical properties Exothermic and Endothermic reactions Calculation of enthalpy changes Calculation of enthalpy changes Investigation 8, titration practical sheet(sodium carbonate and nitric acid), using the burette sheet Atomic orbitals ppt, electron configuration chart, significant figures and isotopes handout Mass spectrometer ppt, You tube clip, labeling mass spectrometer sheet Line spectra ppt, 6: Kinetics (5 hours) 7: Equilibrium (5 hours) 8: Acids and bases (6 hours) 9: Oxidation and reduction (7 hours) 10: Organic chemistry (12 hours) Calculation of enthalpy changes Hess’s Law Hess’s Law Bond enthalpies Bond Enthalpies Rates of reaction Rates of reaction Collision theory Collision theory Collision theory Dynamic equilibrium The position of equilibrium The position of equilibrium The position of equilibrium The position of equilibrium Theories of acids and bases Theories of acids and bases Properties of acids and bases Strong and weak acids and bases Strong and weak acids and bases The pH scale Introduction to oxidation and reduction Introduction to oxidation and reduction Redox equations Reactivity Voltaic cells Electrolytic cells Electrolytic cells Introduction Introduction Introduction Introduction Alkanes Alkanes Alkenes Alkenes Alchohols Halogenoalkanes Organic chemistry ppt, 11: Measurement and data processing (2 hours) Opt B: Human biochemistry (15 hours) Opt E: Environmental Chemistry (15 hours) Halogenoalkanes Reaction pathways Uncertainty and error in measurement Uncertainties in calculated results and graphical techniques Energy (0.5 hours) Proteins Proteins Proteins Carbohydrates Carbohydrates Carbohydrates Lipids Lipds Lipids (+0.5 hours) Air pollution Air pollution Acid deposition (+0.5 hours) Greenhouse effect (+0.5 hours) Ozone depletion (+0.5 hours) Dissolved oxygen in water (+0.5 hours) Water treatment Water treatment (+0.5 hours) Soil Soil (+0.5 hours) Waste Waste You also need to add in another 30 hours of practical work into this plan. I haven´t added it on because I assume you will be doing it as you go through the course and not as a separate block of 30 hours! Feel free to merge/change the lesson titles as required. I have just used the outline straight from the IB subject guide but obviously you will have specific lessons as opposed to the general titles I have given here! Also, if you cover something from one topic in another – just merge the rows as required. This is simply a guideline to help formulate your scheme of work – do feel free to manipulate the plan to suit yourself!