Chapter 3
advertisement

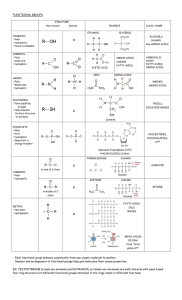
Answers to Review Questions - Chapter 3 1. Sketch the following functional groups: methyl, amino, carbonyl, hydroxyl, carboxyl, and phosphate - use non-ionized forms where appropriate. See Figure 3-1 for drawings of these functional groups. 2. How is the fact that a group is nonpolar, polar, acidic, or basic related to its hydrophilic or hydrophobic properties? Polar functional groups have an electronegative oxygen atom and are therefore hydrophilic. Nonpolar functional groups are hydrophobic. Acidic and basic functional groups are both hydrophilic, as they ionize slightly (i.e. are ions at body pH). 3. Why is the equivalent of a water molecule important to both condensation reactions and hydrolysis reactions? Most monomers are small molecules with a free hydroxyl (OH) or H atom, and polymers are formed by cleaving these off by enzymatic action, forming water in the process (condensation reactions). Degradation of large molecules requires the input of the hydroxyl and the hydrogen from splitting water (hydrolysis). 4. Define monosaccharide, disaccharide, and polysaccharide. Give an example of each. Monosaccharides are simple sugars containing 3-7 carbons - it cannot be degraded by hydrolysis to any other simple sugar. Examples (any ONE of these) glucose, fructose, ribose, galactose, deoxyribose, glyceraldehydes, dihydroxyacetone Disaccharides are the preferred form of transport for simple sugars, and consist of two monosaccharides. Examples - lactose, maltose, sucrose Polysaccharides are macromolecules consisting of repeating units of simple sugars (3 or more), usually glucose. Examples (any ONE of these) - starch, cellulose, glycogen. 5. How does the primary structure of a polypeptide influence its secondary and tertiary structures? The primary structure is the amino acid sequence. The sequence of the amino acids determines whether the protein will take on an alpha-helix or beta-pleated sheet (or both) in its secondary structure. Likewise, the R groups of adjacent amino acids result in a variety of interactions that forms the tertiary structure of a protein. 6. How can the conformation of a protein be disrupted? Denaturation is a disruption of the secondary, tertiary, and/or quaternary structure of a protein by an external factor such as temperature or pH. As the correct molecular conformation unravels, the functioning of the protein is also dramatically affected. 7. Describe saturated, unsaturated, and trans fatty acids. Saturated fats have the maximum possible number of hydrogen atoms, resulting in a carbon chain with all single bonds and a linear form. Unsaturated fats have one or more adjacent double bonds between carbon atoms, resulting in a chain that is non-linear (i.e. "kinked". When unsaturated fats are artificially hydrogenated, the double bonds result in a trans configuration, and therefore mimic the properties of saturated fatty acids. 8. Why do phospholipids form lipid bilayers in aqueous conditions? Phospholipids are amphipathic molecules, with a hydrophilic glycerol end and a hydrophobic fatty acid end, and so naturally form a bilayer with the hydrophobic tails all in the middle, with the hydrophilic glycerol portions oriented out in either direction, interfacing with the aqueous medium.