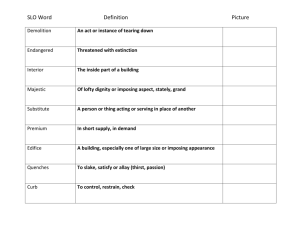
Student Learning Objective (SLO) Template 10/2/13 This template
advertisement

Student Learning Objective (SLO) Template 10/2/13 This template should be completed while referring to the SLO Template Checklist. Teacher Name: ________________Content Area and Course(s): Chemistry Grade Level(s): 11/12 Academic Year: 2013-2014 Please use the guidance provided in addition to this template to develop components of the student learning objective and populate each component in the space below. Baseline and Trend Data What information is being used to inform the creation of the SLO and establish the amount of growth that should take place? Students in this course will be receiving baseline data from a DAP pretest written by APS and used throughout the district. APS teachers were appropriately involved in the creation of items as well as the alignment process to content statements thus supporting a high validity level of the assessments. Results of the pre-assessment were entered into the SLO scoring template. Student strengths and weaknesses were derived from the preassessment data. General trend data (if available) for my students for this SLO includes: Upon analyzing my student baseline data some strengths and weaknesses include: Student Population Which students will be included in this SLO? Include course, grade level, and number of students. This is an 11th and 12th Grade Chemistry Course. All students will be covered by this SLO. A total of ____students are enrolled. There is/are ____course section(s) covered by this SLO. There are _____students on IEPs and ______students are African-American, __________students are white, ______ students are economically disadvantaged. Describe students in detail – any with prior experience, attendance issues, etc. No students will be excluded from the SLO unless ___________ Students who missed 45 days (excused or unexcused) will not be included. Interval of Instruction What is the duration of the course that the SLO will cover? Include beginning and end dates. The Akron Public Schools session for this course duration is one school year from August 28, 2013 to June 5, 2014. Students receive a minimum of one period of science instruction daily. The SLO assessment for fall benchmarking will be administered the week of October_____, 2013 and the spring benchmarking will be administered the week of March _____, 2014. Strategic monitoring, data, and evidence collection will occur between October 2013 and March 2014. Standards and Content What content will the SLO target? To what related standards is the SLO aligned? Chemistry Syllabus and Model Curriculum Course Description Chemistry is a high school level course, which satisfies the Ohio Core science graduation requirements of Ohio Revised Code Section 3313.603. This section of Ohio law requires a three-unit course with inquiry-based laboratory experience that engages students in asking valid scientific questions and gathering and analyzing information. This course introduces students to key concepts and theories that provide a foundation for further study in other sciences as well as advanced science disciplines. Chemistry comprises a systematic study of the predictive physical interactions of matter and subsequent events that occur in the natural world. The study of matter through the exploration of classification, its structure and its interactions is how this course is organized. Investigations are used to understand and explain the behavior of matter in a variety of inquiry and design scenarios that incorporate scientific reasoning, analysis, communication skills and real-world applications. An understanding of leading theories and how they have informed current knowledge prepares students with higher order cognitive capabilities of evaluation, prediction and application. Science Inquiry and Application During grades 9 through 12, all students must use the following scientific processes with appropriate laboratory safety techniques to construct their knowledge and understanding in all science content areas: • Identify questions and concepts that guide scientific investigations; • Design and conduct scientific investigations; • Use technology and mathematics to improve investigations and communications; • Formulate and revise explanations and models using logic and evidence (critical thinking); • Recognize and analyze explanations and models; and 2|Page • Communicate and support a scientific argument. Course Content Structure and Properties of Matter • Atomic structure o Evolution of atomic models/theory o Electrons o Electron configurations • Periodic table o Properties o Trends • Intramolecular chemical bonding o Ionic o Polar/covalent • Representing compounds o Formula writing o Nomenclature o Models and shapes (Lewis structures, ball and stick, molecular geometries) • Quantifying matter • Phases of matter • Intermolecular chemical bonding o Types and strengths o Implications for properties of substances Melting and boiling point Solubility Vapor pressure Interactions of Matter • Chemical reactions o Types of reactions o Kinetics o Energy o Equilibrium 3|Page o Acids/bases • Gas laws o Pressure, volume and temperature o Ideal gas law • Stoichiometry o Molar calculations o Solutions o Limiting reagents • Nuclear Reactions o Radioisotopes o Nuclear energy Assessment(s) What assessment(s) will be used to measure student growth for this SLO? The assessment that will be used is the district-created DAP. The DAP assessment is a pre-post developed by a group of APS teachers. DAP items are aligned directly to Ohio’s New Learning Standards in Science. Each assessment will consist of 56 multiple choice items that span the breadth of content and including stretch in the cognitive demand of items. The assessment does include stretch. It has items that are taught throughout the year giving students the opportunity to demonstrate stretched learning that they have achieved. It also will contain items that students may already have learned. Growth Target(s) Considering all available data and content requirements, what growth target(s) can students be expected to reach? Akron Public Schools will use Effect Size as the measure to determine expected growth. Effect Size is the Post-DAP score minus the Pre-DAP score divided by the mean standard deviation. The Effect Size of 0.40 will be used as the measure that reflects a year’s growth. Rationale for Growth Target(s) What is your rationale for setting the above target(s) for student growth within the interval of instruction? To come up with a measure of what expected progress should be, we have used two main considerations. a. 4|Page When we look at many major longitudinal databases (PIRLS, PISA, TIMSS, NAEP, NAPLAN), they all lead to a similar estimate of an effect size of 0.4 for a b. year’s input of schooling. The average of 800+ meta-analyses based on 240 million students shows an average intervention of 0.40. Therefore an effect greater than 0.40 is seen as above the norm and leading towards a more than expected growth over a year. It is the mission of Akron Public Schools to "ensure that each student in our diverse population achieves his or her fullest potential in a safe and affirming learning center characterized by an extensive, student-focused collaboration of all segments of the community, with an emphasis on preparing students to live and excel in a global environment.” YOU MAY PUT YOUR SCHOOL’S MISSION STATEMENT HERE_______ 5|Page