Question 1.1 - ChemConnections
advertisement

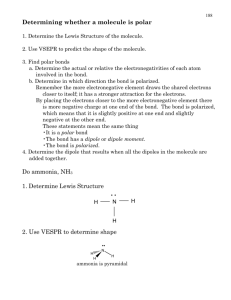
~ 0.1 nm Chapter 1 Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10 -4 microns = 10 -8 centimeters Acids and Bases Nucleus = 1/10,000 of the atom • 1 nm = 10 Å • An atom vs. a nucleus ~10,000 x larger Question 1.1 • What is the electronic configuration of carbon and how many valence electrons does carbon have? A) 1s2 2s2 2px2 (6 valence e-) B) 1s2 2s2 2px1 2py12pz0 (4 valence e-) C) 1s2 2s2 2px12py12pz1 (3 valence e-) D) 1s2 1px1 1py12s2 (4 valence e-) E) 1s2 2s2 2px2 (4 valence e-) Electron Configurations Noble Gases and The Rule of Eight • When two nonmetals react to form a covalent bond: They share electrons to achieve a Noble gas electron configuration. • When a nonmetal and a metal react to form an ionic compound: Valence electrons of the metal are lost and the nonmetal gains these electrons. G.N. Lewis Photo Bancroft Library, University of California/LBNL Image Library Footnote: G.N. Lewis, despite his insight and contributions to chemistry, was never awarded the Nobel prize. Notes from Lewis’ Lewis ’s notebook and his “Lewis Lewis”” structure. Ionic Compounds • Ionic compounds are formed when electron(s) are transferred. • Electrons go from less electronegative element to the more electronegative forming ionic bonds. Covalent Compounds •Share electrons. •1 pair = 1 bond. •Octet rule (“duet” for hydrogen) •Lewis structures: Notice the charges: In one case they balance, can you name the compound? In the other they do not, can you name the polyatomic ion? More about “formal” charge to come. Question 1.2 • Select the correct Lewis structure for methyl fluoride (CH3F). • A) B) • • C) D) Important Bond Numbers (Neutral Atoms!) one bond two bonds three bonds H F Cl O N four bonds Question 1.3 • What is the correct Lewis structure of formaldehyde (H2CO)? • A) B) • C) D) Br C Question 1.4 • Which of the following contains a triple bond? • A) SO2 • B) HCN • C) C2H4 • D) NH3 I Formal Charge • • • HNO3 Nitric Acid Equals the number of valence electrons of the free atom minus [the number of unshared valence electrons in the molecule + 1/2 the number of shared valence electrons in the molecule]. Moving/Adding/Subtracting atoms and electrons. See examples on the board. Formal charge = number of valence electrons – (number of lone pair electrons +1/2 number of bonding electrons) Question 1.5 • What is the formal charge of the sulfur atom in the Lewis structure? • A) -1 • B) 0 • C) +1 • D) +2 Resonance Theory Whenever a molecule or ion can be represented by more than 1 valid Lewis structure where the difference is only in the positions of the electrons not atoms, then: 1. No single resonance structure is a correct one for it. 2. Instead, the actual molecule or ion will be a hybrid or weighted average of these structures which are not all equal. There contribution varies. 3. See Table 1.6 pp. 27-28 Therefore, Resonance Structures exist only in theory and are mental constructs, which are very important and useful for predicting chemical behavior and chemical reactivity nevertheless. Resonance( Resonance ) ≠ Equilibrium ( ) Question 1.6 •How many resonance structures can be written for the NO3- ion in which the nitrogen atom bears a formal charge of +1? A) 1 B) 2 C) 3 D) 4 E) 5 VSEPR Model Valence Shell Electron Pair Repulsion Question 1.7 • Which resonance structure contributes more to the hybrid? VSEPR • A) B) VSEPR Model Predicting a VSEPR Structure The molecular structure of a given atom is determined principally by minimizing electron pair (bonded &free) repulsions through maximizing separations. Some examples of minimizing interactions. Orbital Geometry Molecular Geometry Bond Angle # of lone pairs Linear Linear 0 Trigonal Planar Trigonal Planar 0 Trigonal Planar Bent 1 Tetrahedral 0 Tetrahedral Trigonal Pyramidal 1 Tetrahedral Bent 2 Trigonal Bipyramidal Trigonal Bipyramidal 0 Trigonal Bipyramidal Seesaw 1 Trigonal Bipyramidal T-shape 2 Trigonal Bipyramidal Linear 3 Octahedral Octahedral 0 Octahedral Square Pyramidal 1 Octahedral Square Planar 2 Chem 226 Tetrahedral • 1. Draw Lewis structure. • 2. Put pairs as far apart as possible. • 3. Determine positions of atoms from the way electron pairs are shared. • 4. Determine the name of molecular structure from positions of the atoms. Lewis Structures / VSEPR / Molecular Models • Computer Generated Models Ball and stick models of ammonia, water and methane. For many others see: http://chemconnections.org/organic/pdb-lib/ http://chemconnections.org/organic/chem226/Smell-Stereochem.html Covalent Compounds •Equal sharing of electrons: nonpolar covalent bond, same electronegativity (e.g., H2) • Unequal sharing of electrons between atoms of different electronegativities: polar covalent bond (e.g., HF) Question 1.8 • Which of the following bonds is the most polar? • A) B) • C) D) Bond Dipole & Dipole Moment • Dipole moments are experimentally measured. • Polar bonds have dipole moments. dipole moment (D) = µ = e x d (e) : magnitude of the charge on the atom (d) : distance between the two charges Question 1.9 • Which of the following bonds have the greatest dipole moment? • A) B) • C) D) Bond Polarity A molecule, such as HF, that has a center of positive charge and a center of negative charge is polar, and has a dipole moment. The partial charge is represented by δ and the polarity with a vector arrow. H F δ+ Question 1.10 • In which of the compounds below is the δ+ for H the greatest? • A) CH4 • B) NH3 • C) SiH4 • D) H2O δ− Question 1.11 Electrostatic Potential Maps Models that visually portray polarity and dipoles • In which of the following is oxygen the positive end of the bond dipole? • A) O-F • B) O-N • C) O-S • D) O-H Hydrogen Halides Molecular Polarity & Dipole Moment When identical polar bonds point in opposite directions, the effects of their polarities cancel, giving no net dipole moment. When they do not point in opposite directions, there is a net effect and a net molecular dipole moment, designated δ. Molecular Dipole Moment The vector sum of the magnitude and the direction of the individual bond dipole determines the overall dipole moment of a molecule An electrically charged rod attracts a stream of chloroform but has no effect on a stream of carbon tetrachloride. Ammonia and in the Ammonium Ion Water The “Lotus Effect” Polarity & Physical Properties Biomimicry Ozone and Water http://www.bfi.org/Trimtab/spring01/biomimicry.htm 0.1278 nm • • • Resultant Molecular Dipoles > 0 Solubility: Polar molecules that dissolve or are dissolved in like molecules • • • The Lotus flower Water & dirt repellancy Wax Lotus petals have micrometer-scale roughness, resulting in water contact angles up to 170° See the Left image in the illustration on the right. The “Lotus Effect” Question 1.12 Biomimicry http://www.sciencemag.org/cgi/content/full/299/5611/1377/DC1 • • • Isotactic polypropylene (i-PP) melted between two glass slides and subsequent crystallization provided a smooth surface. Atomic force microscopy tests indicated that the surface had root mean square (rms) roughness of 10 nm. A) The water drop on the resulting surface had a contact angle of 104° ± 2 B) the water drop on a superhydrophobic i-PP coating surface has a contact angle of 160°. • Which molecule would have a dipole moment equal to zero? A) B) C) D) CCl4 CH3OH CH3OCH3 CH3Cl Science, 299, (2003), pp. 1377-1380, H. Yldrm Erbil, A. Levent Demirel, Yonca Avc, Olcay Mert Molecular Representations VIDEO Empirical Formula, Molecular Formula, Structure: (Lewis, Kekule, Condensed, Line), Visual Model: wireframe, stick, ball & stick, space filling, electrostatic, energy surface Question 1.13 Bond Line OH • The bond-line representation for (CH3)2CHCH2CH2CHBrCH3 is Molecular Formula C7H16O • A) B) • C) D) Question 1.14 Question 1.15 • The total number of bonded pairs of electrons and of unshared pairs of electrons in morpholine is: • A) 7, 0 • B) 7, 1 • C) 15, 0 • D) 15, 1 • E) 15, 3 • The molecular formula of morpholine is: • A) C2HNO • B) C4HNO • C) C4H4NO • D) C4H5NO • E) C4H9NO Question 1.16 C2H6O: H H H C C H H H H H O C H Ethanol (b.p. 78.5 C) • How many constitutional isomers can have the formula C3H7Cl? • A) one • B) two • C) three • D) four H O C H H Dimethyl ether (b.p. –23 C) Constitutional or Structural Isomers: These isomers have the same molecular formula, but different arrangement of atoms in space (Bonding differences) Question 1.17 O O H O OH O O CH3 CHO • How many constitutional aldehyde isomers can have the molecular formula C4H8O? • A) one • B) two • C) three • D) four Line Drawing and Ball & Stick More Molecular Representations Empirical Formula, Molecular Formula, Structure: (Lewis, Kekule, Condensed, Line), Visual Model: wireframe, stick, ball & stick, space filling, electrostatic, energy surface 8.16 Å (0.816 nm) http://chemconnections.org/organic/chem226/Smell-Stereochem.html Worksheet: Organic Molecules I http://chemconnections.org/organic/chem226/ Very Large Molecules Very Large Molecules:DNA http://www.umass.edu/microbio/chime/beta/pe_alpha/atlas/atlas.htm Views & Algorithms http://info.bio.cmu.edu/courses/03231/ProtStruc/ProtStruc.htm B-DNA: Size, Shape & Self Assembly 46 Å 10.85 Å 10.85 Å Rosalind Franklin’s Photo 12 base sequence Several formats are commonly used but all rely on plotting atoms in 3 dimensional space; .pdb is one of the most popular. (1953-2003) http://molvis.sdsc.edu/pdb/dna_b_form.pdb Atomic Orbitals Atomic Orbitals s orbitals p orbitals Molecular Orbitals • Atomic orbitals mix to form molecular orbitals • σ bond: formed by overlapping of two s orbitals Molecular Orbitals (MO) MO: a linear combinaiton of AOs H2: Chemical bonds formation---AO’s overlapping Ψ2,1 = Ψ1sA(1)Ψ1sB (2 ) + Ψ1sB (1)Ψ1sA(2 ) Bond formation: Ψ1, 2 = Ψ1sA(1)Ψ1sB (2 ) − Ψ1sB (1)Ψ1sA(2 ) 1.Closed energy level 2.Similar size and shape of AOs 3.Significant overlapping In-phase overlap of s atomic orbitals form a bonding MO; out-of-phase overlap forms an antibonding MO A single bond is a sigma (σ) bond. A sigma bond (σ) is formed by end-on overlap of two p orbitals Double bonds have 1 π and 1 σ bond. A π bond is weaker than a σ bond. A double bond is shorter and stronger than a single bond. A pi bond (π) is formed by sideways overlap of two parallel p orbitals. A π bond is weaker than a σ bond. A double bond is shorter and stronger than a single bond. Bonding in Methane and Ethane: Single Bonds Hybridization of orbitals: The orbitals used in bond formation determine the bond angles • Tetrahedral bond angle: 109.5° Hybrid Orbitals of Ethane The sigma bond (σ) between the carbon atoms is part of the overall hybridization. The molecular orbital allows rotation about the C-C single bond. • Electron pairs spread themselves into space as far from each other as possible Bonding in Ethene Carbon Double Bonds Double bonds have 1 π and 1 σ bond. A π bond is weaker than a σ bond. A double bond is shorter and stronger than a single bond. The pi bond (π) of the sp2 hybrid does not allow rotation about the double bond. This produces a fixed geometry about the double bond and results in cis-trans, (Z-E), isomerism • The bond angles of the sp2 carbon are about 120° • The sp2 carbon is trigonal planar • The atoms bonded to the sp2 carbon are all in the same plane Bonding in Ethyne: A Triple Bond Question 1.18 • In which of the following compounds could be found the shortest carbon- carbon bond(s)? • A triple bond consists of one σ bond and two π bonds •Triple bonds are shorter and stronger than double bonds • There is a bond angle of the sp carbon: 180 ° A) C3H8 B) C4H10 C) C3H4 D) C3H6 Question 1.19 • What is the molecular shape about the carbon atoms of acetylene (HC≡CH)? • • • • A) B) C) D) tetrahedral bent trigonal planar linear Reactive Intermediates Carbocation Reactive Intermediates Radical Reactive Intermediates Carbanion