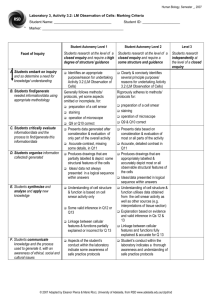
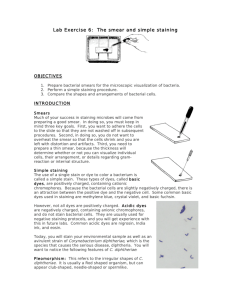
Smear Preparation and Simple Stain Objectives: Make bacterial
advertisement

Smear Preparation and Simple Stain Objectives: Make bacterial smear slides (usually called smears) Distinguish cells on these slides using a simple stain procedure Unstained microbial cells are nearly transparent when observed by light microscopy and thus are difficult to see. Effective use of a microscope requires bacteria to be mounted on a slide and stained. However, in order to stain microorganisms, a smear preparation must first be made. In our laboratory a smear preparation always consists of three steps: 1) A SPREAD of cells 2) AIR DRYING the slide thoroughly 3) HEAT FIXING with a flame Remember these three steps in the smear preparation. A bacterial smear is a dried preparation of bacterial cells on a glass slide. You may make a smear from a dry culture (from a slant, stab or plate), or from a wet/liquid culture. In order to be useful, a smear must have the following qualities: 1. The bacteria must be spread evenly over the slide and be concentrated enough to be located, but dispersed enough so that their natural arrangement can be seen. Ideally a single cell layer will be achieved; dense areas (i.e. multiple cell layers) on a slide is often not useful for examination. 2. The bacteria must be adequately fixed to the slide so they don’t wash off during normal washing procedures in staining. 3. The bacteria must not be deformed so that their original morphology can be observed. In our lab we will work with primarily three bacterial species: Bacillus subtilis (“sneaky rod”) Escherichia coli (colon bacteria named after Mr. Escherichia) Staphylococcus epidermis (bunch of grapes) Smear procedure I. CLEANING MICROSCOPE SLIDES Slides are packed with a thin film of oil on them. Clean, grease-free slides are essential for obtaining good stained preparations. Before beginning any smear or staining procedure, slides should be cleaned as follows: 1. Wet the tip of your index finger and rub it on some abrasive cleanser (e.g., Bon Ami. Comet, Ajax, etc.). 2. Holding the slide by the sides, spread the paste over both surfaces of the slide. 3. Wash the slide thoroughly with running water, first rubbing away the cleanser, then just letting water flow over the slide. 4. To dry the slides you may rinse them in a shallow isopropanol bath for a few seconds OR you may place them on their sides in a 37’C incubator. 5. Flame the "up" side of the slide for a moment with a candle. Allow it to cool. 6. Store cleaned slide right side up between sheets of a folded paper towel or in slide box. NOTE: You will be using around 1-2 dozen slides over the quarter- you can clean several slides now and store them in a slide box for use later. Simply put a small piece of tape on the box and store it in a cupboard of near your bench. NOTE: It is always a good idea to prepare at least two smear slides to have a backup if one fails. If you are preparing multiple slide smears, set up each slide individually. Once a smear is drying, you can set up another slide so that multiple smears can dry at one time. Often one slide will have several smears on it (they can be fairly small in area). You should label an end of your slide before you set up your smears so you (and everyone else) will know what’s going on. When you begin staining, stain one slide at a time, i.e., complete the entire staining protocol for one slide before beginning another. Timing can be critical and if you attempt to run an assembly line you run the risk of ruining all of your slides. All slides must be labeled. You may write directly on the slide with your marker (remember to always write in small print). The most important information you need is the date, the sample and your initials. Several problems can result from dirty slides: 1. If all oil is not removed from the slide, then the bacteria may be arranged in concentric circles around the oil that remains on the slide. 2. If all cleanser is not removed from the slide, the stain will attach to it and form a colored haze over the slide. 3. Heat fixing can be ineffective on a dirty slide. II. Smear Preparation from a Solid Culture Smears made from solid cultures are usually better than those made from broth. The most common problem is the use of too much inoculum. Remember to only fill a small edge of your loop with inoculum from the culture. ALWAYS inoculate a dry sample into a loopful or two of water. III. Smear Preparation from a Broth Culture Smear preparations from broth cultures are more difficult for a couple of reasons. One problem is that chemicals in the nutrient broth interfere with adhesion if the bacteria to the slide. Another problem is that broth cultures may contain a lower concentration of bacteria than those on solid media. You may spread a loopful or two of liquid culture directly onto a clean slide- there is no need to add water to the slide first. After resuspending a broth culture, examine it for turbidity (cloudiness). The more cloudy the culture, the higher the concentration of bacteria. If a culture is not very cloudy, you must take a larger sample when making a smear than you would if the culture was very cloudy. You can also concentrate the bacteria by centrifuging the culture tube, aspirating some of the media using sterile technique and resuspending the cells in a smaller volume. IV. Simple Staining A simple stain is application to a smear of a single dye that colors the cell uniformly. The purpose of a simple stain is to highlight the entire population of microorganism(s). A stain is a procedure, the dye(s) and smear(s) used are components of that procedure. Most dyes are salts in which one of the ions is colored, i.e., it reflects light of a specific wave length. This ion is called a chromophore and it will specifically bind to a cell or a structural part of the cell. Methylene blue, for example, is methylene blue chloride (MBCl) which, when ionized, becomes: MB+ (chromophore) + Cl-. The chromophore (methylene blue cation in this example) attaches to negatively charged particles (e.g., DNA- in the nucleus): MB+ plus DNA- equals MBDNA (stained). If the color-bearing ion (chromophore) is positively charged, the stain is basic (it has given up a negative charge). If the chromophore is negatively charged it is acidic (it has given up a positive charge). When methylene blue is used to stain bacteria, the entire bacterial cell is colored blue; this is the essence of a simple stain procedure. Often, as with staining of cheek cells, dyes bind to specific cellular structures. In these cases the dyes are part of a specific or differential stain procedure. Five basic dyes will be used in our laboratory: Basic dye Absorbance maxima (nm) crystal violet 590 safranin 530 carbol fuchsin 544 methylene blue 665 malachite green 621 Acidic dye example Eosin 525 V. Procedure A. Smear preparation from a solid culture 1. Add 1-2 loops of water to your slide 2. Flame the inoculating loop and cool for 30 seconds 3. Touch the growth on the plate or slant with one side of the loop. Do not scrape or dig into the agar. If you dig into the agar, it will mix with the inoculum. Chunks of agar will cause cells to clump and the chunks will absorb stain. 4. Very briefly touch the side of the loop with the cells to the water and move it back and forth a couple of times. 5. Lay your loop flat and spread the cells to an area just smaller than the size of a dime. 6. Resterilize the inoculating loop and set it aside or continue on to another inoculum and smear. 7. Allow the smear to air dry (don’t use high heat). When dry, the smear may appear faintly cloudy or very little may be seen depending upon the amount of growth in the tube. 8. The smear must be "heat fixed" to help the cells adhere to the slide and not be washed off during the gentle rinses of staining procedures. This is accomplished by passing the slide (smear side up) back and forth through the flame of the candle slowly two times (these should be about 1 second passes: “one Mississippi”). Do not overheat the slide. You should be able to touch the underside of the slide comfortably to the back of your hand. When the bacteria become too hot during the fixing process, they become distorted. NOTE: Heat fixed bacteria may be washed off the slide with excessive or vigorous washing. In our staining procedures we use rapid rinses of water poured gently from a plastic beaker. 9. Once smear is complete, proceed to simple staining protocol below. You may accumulate smear preps prior to proceeding to staining. B. Smear from a broth culture 1. Disperse the bacteria that have settled to the bottom of the culture tube by swirling/flicking the tube gently. 2. Flame the inoculating loop and cool for a few seconds. 3. Transfer 1-2 loops of culture to a previously labeled slide. There is NO NEED to add water to the slide first. 4. Spread the suspension out to approximately the size of a dime as above. 5. Resterilize the inoculating loop and set aside. 6. Continue with smear preparation as described above (steps 7-9) C. Simple Stain 1. Flood your smear with methylene blue or crystal violet stain, allow it to remain for 15 seconds, and then rinse gently with water. (Never apply the running water directly onto the smear. Hold the slide at an angle over your rinse pan, allowing the water to make first contact with one end of the slide and wash over the smear.) NOTE: cover only the smear with staining solution- DO NOT cover the entire slide (this is wasteful). 2. Tamp down the edge of the slide onto paper towels, gently blot off excess water and dry the smear on your bench in air. Do not rub slide with paper as this will mechanically force cells off the slide. 3. Examine your smear under the microscopes. Cover glasses are not used to protect smears commonly produced in the laboratory. If you wish to save your slide, carefully blot off any oil with a Kim wipe folded twice. Although this will remove some of the cells from the smear, if you are careful, these slides can be viewed 3-4 times before they are no longer useful. Obtain a slide box and use tape to label it with your name. 4. Draw representative cells. From this simple procedure what do you now know about the species of bacteria you used today? A table would be helpful here- and useful throughout the quarter. 5. If a problem exists, check with the Instructor and repeat from start to finish using the same culture. Change only one step and see what effect this one change makes. Study Guide 1. What are three qualities of an effective and useful bacterial smear? 2. What would a slide look like if it had been improperly cleaned? 3. What would happen to the smear if you failed to “heat fix” the slide before staining? 4. Define and give an example of a basic simple stain. Be sure your definition is complete! 5. Draw and label your smears in your lab journal. Draw a circle that represents your field of view and draw the bacteria to scale. Your label should include morphology, arrangement, and source (solid or broth culture). 6. Evaluate the quality of your smears for cleanliness and density. Are there any modifications you should make in your technique? Useful throughout the course will be a species table. Column headers are the species used in the lab (primarily three) and row headers are characteristics of those species such as cell shape, culture color, reaction to particular stains, etc. An example of a partial table is shown below (more should be added as more information comes in). I’ve given you optimum growth temperatures for these species. Bacillus subtilis Cell shape Growth temperature TSA colony color TSA colony odor Etc… 30’C Escherichia coli 37’C Staphylococcus epidermis 37’C