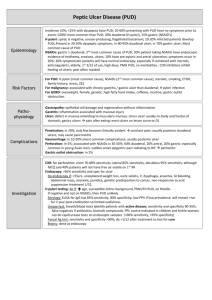
GASTROINTESTINAL DISORDERS
advertisement

SECTION FIVE GASTROINTESTINAL DISORDERS Robin L. Corelli SECTION EDITOR CHAPTER 27 Upper Gastrointestinal Disorders John K. Siepler, Candace Smith-Scott DYSPEPSIA 27-2 Management Strategies 27-3 Treatment Recommendations 27-3 PEPTIC ULCER DISEASE 27-3 Epidemiology 27-3 Incidence and Prevalence 27-3 Geographic Variation 27-4 Gender 27-4 Age 27-4 Morbidity and Mortality 27-4 Physiology of the Upper Gastrointestinal Tract 27-4 Pathogenesis 27-5 Helicobacter pylori 27-5 Risk Factors 27-6 Clinical Presentation 27-6 Treatment 27-6 H2-Antagonists 27-6 Mechanism of Action 27-6 Efficacy 27-6 Potency 27-7 Pharmacokinetics 27-8 Adverse Effects 27-8 Drug Interactions 27-8 Dosing 27-9 Proton Pump Inhibitors 27-9 Mechanism of Action 27-9 Efficacy 27-9 Pharmacokinetics 27-10 Adverse Effects 27-10 Drug Interactions 27-10 Sucralfate 27-10 Mechanism of Action 27-10 Efficacy 27-10 Adverse Effects 27-10 Drug Interactions 27-10 Antacids 27-10 Mechanism of Action 27-10 Efficacy 27-11 Adverse Effects 27-11 Drug Interactions 27-11 Eradication of H. pylori 27-11 Monotherapy 27-11 Combination Therapy 27-11 Recommended Combination Regimens 27-12 Detection of Helicobacter 27-13 Drug-Induced Peptic Ulcer Disease 27-15 Etiology and Clinical Presentation 27-15 Mechanism of Action 27-15 Treatment 27-16 H2-Receptor Antagonists 27-16 Proton Pump Inhibitors 27-16 ZOLLINGER-ELLISON SYNDROME 27-17 Pathogenesis 27-17 Clinical Presentation 27-17 Upper gastrointestinal (GI) disorders encompass a variety of conditions that can cause gastric discomfort, including dyspepsia, peptic ulcer disease (PUD), and gastroesophageal reflux disease (GERD). This chapter addresses four topics in the following order: dyspepsia, PUD (including Zollinger-Ellison [ZE] syndrome), GERD, and prophylaxis of stress-related mucosal lesions. The term dyspepsia refers to symptoms thought to originate in the upper GI tract and is used commonly to refer to persis- Diagnosis 27-17 Treatment 27-17 Proton Pump Inhibitor Administration 27-18 GASTROESOPHAGEAL REFLUX DISEASE 27-18 Pathogenesis 27-19 Clinical Presentation 27-19 Treatment 27-19 Goals 27-19 Lifestyle Changes 27-19 Drug Therapy 27-20 Antacids 27-20 H2-Antagonists 27-20 Proton Pump Inhibitors 27-20 Sucralfate 27-20 Metoclopramide 27-20 Maintenance Therapy 27-21 STRESS-RELATED MUCOSAL BLEEDING 27-21 Pathogenesis 27-21 Risk Factors 27-21 Treatment 27-22 H2 -Antagonists 27-22 Sucralfate 27-22 Proton Pump Inhibitors 27-22 tent or recurrent pain or discomfort centered in the upper abdomen.1 This pain includes ulcer-like, GERD-like, and dysmotility-like discomfort. Discomfort is defined as a subjective, unpleasant feeling that the patient does not interpret as pain but that can include symptoms such as fullness, bloating, or nausea.1,2 It is estimated that 25% to 55% of the U.S. population will experience some form of dyspepsia in their lifetime (Fig. 27-1). Dyspepsia of some kind accounts for approximately 60% of all upper GI complaints in the U.S. population. 27-1 27-2 • GASTROINTESTINAL DISORDERS U.S. Population Dyspepsia 25–55% (<50% H. pylori positive) Peptic ulcer 6–15% (85–90% H. pylori positive) Asymptomatic 45–74% (~25% H. pylori positive) Nonulcer dyspepsia or gastritis 20–40% (~50% H. pylori positive) FIGURE 27-1 Prevalence of dyspepsia and peptic acid disease in the United States. Approximately 4% to 10% of all Americans will develop a peptic ulcer during their lifetime, and the prevalence of active disease is approximately 3%.3 The incidence of PUD varies with the type of ulcer (e.g., gastric or duodenal), geographic location, gender, age, and a variety of environmental factors. Race, socioeconomic status, and psychological stress do not correlate with the development of PUD.4 As discussed in more detail later, three factors stand out as being the most important to the development of PUD: bacterial GI infection with Helicobacter pylori (H. pylori), ingestion of nonsteroidal anti-inflammatory drugs (NSAIDs), and cigarette smoking.4 It is estimated that up to 90% of peptic ulcers, in the absence of NSAID exposure, are associated with H. pylori infection. However, one should avoid the false impression that the presence of H. pylori colonization is an indication that an ulcer will develop. As illustrated in Figure 27-2, a high proportion (approximately 40%) of the general population tests positive for H. pylori colonization, but only 15% to 30% of those with positive test results will develop PUD at some time in their life. GERD is defined as the presence of heartburn two or more times weekly that is associated with a change in lifestyle.5 It is associated with a backflow (i.e., reflux or regurgitation) of GI contents into the esophagus resulting in heartburn and regurgitation symptoms. The classic symptom of reflux is “heartburn,” which is described commonly either as a pain in the center of the chest (which may mimic cardiac angina), or as a sensation of “burping up” stomach contents into in the mouth. Patients with chronic, persistent heartburn are more likely to develop esophageal adenocarcinoma.6 Esophagitis is associated with GERD and is the presence of inflammation in the esophagus. The most common causes of GERD are inappropriate lower esophageal sphincter (LES) relaxation and reduced resting pressure in the sphincter: both facilitate the regurgitation of acid from the stomach into the esophagus. Although it is normal for the esophageal and lower esophageal sphincters to relax to allow food passage, relaxation of these sphincters at other times is abnormal. A hiatal hernia (a defect in the diaphragm that allows the stomach to slide into the chest cavity) does not cause GERD but may exacerbate the situation by decreasing the ability of gastric acid from draining back through the sphincter because of differences in the pressure gradient within the abdomen. Some drugs (e.g., verapamil, theophylline) also may cause the lower esophageal sphincter to relax. Terminology can sometimes be misleading; therefore, think of GERD as a disease, heartburn as a symptom, and esophagitis as an endoscopic finding. These disorders are not mutually exclusive, and effective treatment of one disorder does not guarantee that the patient will be symptom free. This principle is most relevant to the treatment of PUD. With the realization that peptic ulcer often has an infectious cause, it is now possible to talk of treatment in terms of “cure” of the ulcer after antibiotic therapy, not just symptom relief or temporary healing. The presence of continued symptoms could indicate coexistence of nonulcer dyspepsia, GERD, or both. DYSPEPSIA Dyspepsia is usually the main complaint for patients who present with upper GI problems. As defined above, dyspepsia occurs in approximately 26% of the population in the United States and in as many as 41% in England.1,2 As dyspepsia is a symptom complex that may apply to several patient conditions, the exact cause can be one or several different disorders. Once investigated, approximately 50% of patients have a specific problem (e.g., PUD or GERD). In the remainder, no cause is often discovered. There have been several attempts to define dyspepsia,1,7 and it can be classified into several subclassifications which attempt to associate the type of dyspepsia with a cause. Patients who present with dyspepsia but have not undergone any diagnostic tests are said to have “uninvestigated” dyspepsia. In addition, patients with a heartburn-like dyspepsia are said to have “GERD-like” dyspepsia, those with dyspepsia that is U.S. Population H. pylori positive: 40% Peptic ulcer 15–30% H. pylori negative: 60% Nonulcer Asymptomatic Peptic ulcer Nonulcer Asymptomatic dyspepsia or 30–65% Rare dyspepsia or 60–80% gastritis gastritis 20–40% 20–40% FIGURE 27-2 Relationship of Helicobacter pylori to peptic ulcer and nonulcer dyspepsia. UPPER GASTROINTESTINAL DISORDERS described as crampy are said to have “dysmotility-like” dyspepsia, and those with abdominal pain that is burning and relieved by meals are said to have “ulcer-like” dyspepsia. Some disagree with the inclusion of heartburn in dyspepsia classifications and would consider dyspepsia to be limited to ulcerlike or dysmotility-like. Despite these classifications, the predictive value of dyspepsia for specific pathology is poor. Understanding these classifications is essential to understanding studies that investigate this problem. When evaluating studies, close attention must be paid to the authors’ definitions and inclusion criteria for study subjects when attempting to review results. Diagnosis for a patient with a new complaint of dyspepsia is usually directed at the suspected cause. Thus, a patient who complains of “GERD-like” dyspepsia will be diagnosed and treated like a patient with GERD, and a patient who complains of “ulcer-like” dyspepsia will be diagnosed and treated like a patient with PUD. Once a patient has undergone diagnostic procedures (usually endoscopy) without finding a specific cause for their dyspepsia, it will be classified as “nonulcer” dyspepsia. The management strategy for patients who have dyspepsia of unknown cause or are classified as having nonulcer dyspepsia varies and has been the subject of numerous studies. For treatment, most of these patients will receive H. pylori testing and eradication, antisecretory medications (either H2antagonists or proton pump inhibitors), or a combination of both. The remainder of this section reviews the efficacy of these strategies. Management Strategies Perhaps the first management option is whether empiric treatment or further diagnosis followed by a specific treatment is the most effective course of action.8 Several trials examined whether H. pylori testing and eradication would provide better symptom control than a strategy that based treatment on results of endoscopy.9–11 The entry criteria for the largest of the three trials included patients who presented to their primary care practitioners with dyspepsia. These patients had truly uninvestigated dyspepsia. Symptoms were controlled better in the endoscopy-directed treatment group. The remaining two trials, which enrolled patients referred for endoscopy, failed to find a difference in symptoms. It is likely that the patients in the two unsuccessful studies were selected by an initial empiric trial by their primary care practitioner.10,11 If the initial treatment strategy is empiric treatment, acid suppression is often the first choice. There are numerous trials that compare the various options in this area. Three trials comparing PPIs and H2-antagonists found patients receiving PPIs to have significantly better symptom control.12–14 In these studies, PPIs appear to offer superior control of heartburn-like dyspepsia. However, the Cochrane Database review concludes that there is insufficient evidence to make the claim that PPIs are superior to H2-antagonists.8 Whereas the role of H. pylori is widely accepted in PUD, its role in other GI conditions such as dyspepsia is not clear. When patients infected with H. pylori are queried, a broad range of symptoms are reported including dyspepsia and • 27-3 heartburn. Until recently, studies designed to determine the role of H. pylori in nonulcer dyspepsia have not studied appropriate outcomes. Recently several studies comparing H. pylori eradication with alternate therapy have been published that used appropriate end points. Most of these trials find no significant difference in symptom resolution between the H. pylori eradication group and the control subjects. However, McColl and colleagues reported a significant improvement in symptoms in nonulcer dyspepsia patients who had H. pylori eradicated (with antisecretory and antimicrobial agents) compared to those receiving 4 weeks of PPI monotherapy.15 This study used a continuous symptom assessment tool that patients recorded for 12 months, but they also included many patients who may have had PUD. While the overall success favored H. pylori eradication, symptom resolution was low and observed in only 27% of patients compared with 12 % for the PPI-treated group. In contrast, Blum and colleagues evaluated H. pylori eradication versus 4 weeks of a PPI and evaluated symptoms at 1 year.16 Control of symptoms was similar in the two groups (26% to 30%). One main difference between the McColl and Blum studies is that Blum excluded patients with a history of peptic ulcer or GERD. This may explain some of the different results as PPIs would be expected to be superior in patients with GERD as their origin of their dyspepsia. It appears that both H. pylori eradication and PPIs provide symptom control in patients with nonulcer dyspepsia. Studies arriving at different conclusions regarding empiric treatment are likely dependent on patient selection. One clear conclusion is that overall symptom control is poor, with less than one-third of patients being free of symptoms with either treatment regimen. Treatment Recommendations Patients with dyspepsia should receive appropriate diagnosis to determine the cause of their symptoms. If the cause is determined to be peptic ulcer, GERD, an NSAID-associated ulcer, or another condition, appropriate treatment should be initiated. If no specific cause is found, it appears that PPIs or H. pylori testing and (if positive) eradication should be tried. As studies demonstrate only modest efficacy, appropriate followup is important. PEPTIC ULCER DISEASE Peptic ulcers are lesions in the stomach or duodenum that occur as a result of the activity of acid and pepsin. ZollingerEllison syndrome (ZES) is a rare form of PUD resulting from hypersecretion as a result of a gastrin secreting tumor. Epidemiology Incidence and Prevalence During the beginning of the 20th century, the incidence of PUD significantly increased, reaching a peak in the early 1950s.17 Over the past two decades, both outpatient episodes of duodenal ulcers and ulcers requiring hospitalization have declined. Whether this decline reflects an actual decrease in the incidence of ulcer disease or the combined influences of more effective therapy, changes in hospital diagnostic 27-4 • GASTROINTESTINAL DISORDERS practices or criteria, and institution coding changes is unclear. Although hospitalization for duodenal ulcers has declined, hospitalization for gastric ulcers has remained stable. The lack of decline in gastric ulcers may be attributable to aging and the use of NSAIDs. Geographic Variation PUD occurs worldwide with significant geographic variation. In Japan, the incidence of gastric ulcer is approximately 5 to 10 times more common than duodenal ulcer; however, in most European countries and the United States, duodenal ulcers are approximately twice as common as gastric ulcers.18 Gender In the United States through the 1970s, more women than men developed PUD.19,20 The incidence of gastric ulcers is approximately the same for men as for women; however, hospitalizations for women with gastric ulcers have increased significantly for patients older than 65.19 Age Gastric ulcers rarely develop before age 40, and the peak incidence occurs from ages 55 to 65.20 In contrast, the incidence of duodenal ulcers increases with age until the age of approximately 60 years.2 The prevalence of H. pylori associated ulcers is low in children, but the incidence also increases with age. Morbidity and Mortality Mortality caused by PUD has declined over the past 20 years. Fewer than 2% of ulcer patients receiving therapy are expected to have a serious complication including bleeding, perforation, or obstruction. Nevertheless, PUD remains one of the most common GI diseases that results in loss of work and high-cost medical services.20 Physiology of the Upper Gastrointestinal Tract The stomach consists of three anatomically and functionally distinct regions: the cardia, the body, and the antrum (Fig. 27-3). The body, which makes up approximately 80% to 90% of the stomach, contains the parietal cells, which secrete acid and intrinsic factor which is required for vitamin B12 absorption. The body of the stomach also contains chief cells, which secrete pepsinogen. The antrum constitutes approximately 10% to 20% of the stomach and contains the G-cells, which secrete the hormone gastrin. Various stimuli can trigger the secretion of acid into the gastric lumen. Neurologic impulses, originating in the central nervous system (CNS) and initiated by the sight, smell, and taste of food, travel along cholinergic pathways to provoke the release of acetylcholine in both the parietal cell and antral Gcell areas (Fig. 27-4). Subsequently, gastric distention stimulates oxyntic and antral receptors, and food protein further stimulates antral receptors to further trigger the release of acetylcholine at nerve endings in parietal and G-cell areas. The acetylcholine in turn stimulates the antral G-cells to release the hormone gastrin. Other stimuli cause secretion of histamine in the vicinity of the parietal cells. All three mediators—acetylcholine, gastrin, and histamine—converge to activate potassium-hydrogen-adenosine triphosphatase (KH-ATPase) located on the parietal cell surface. The secretion of acid occurs against a concentration gradient and therefore requires the K-H-ATPase proton pump.21 As the hydrogen ion concentration increases, it inhibits further secretion of gastrin through a feedback pathway. Cholecystokinin, glucagon, and vasoactive intestinal peptide also inhibit gastrin secretion. Thus, gastric acid secretion is regulated through both positive and negative feedback mechanisms. Pepsinogen, released from the chief cells, is a proenzyme that will form pepsin under acidic conditions (pH 3.5). Pepsin combines with acid to form a proteolytic complex, which further aids in the digestive and ulcerogenic processes.2 Several mechanisms protect the gastroduodenal mucosa from the digestive effects of pepsin and acid. Prostaglandin E and somatostatin, located on the basolateral membrane of the parietal cell, inhibit gastric acid secretion, maintain mucosal blood flow, and stimulate production of mucus and bicarbonate.22The secretion of mucus by superficial epithelial cells and mucous cells throughout the stomach protects against the erosive effects of acid. Gastric mucus is a viscous gel that serves as a mucosal lubricant, a trap for micro-organisms, and a barrier to the back diffusion of hydrogen ions from the mucosa.22 ESOPHAGUS CARDIA Mucus-Secreting Cells DUODENUM PYLORUS BODY Parietal Cells (Acid and Intrinsic Factor) Chief Cells (Pepsinogen) ANTRUM G Cells (Gastrin) FIGURE 27-3 Gastrointestinal anatomic regions. UPPER GASTROINTESTINAL DISORDERS • 27-5 Food (Sight, Smell, Taste) CENTRAL NERVOUS SYSTEM ANTRAL RECEPTOR OXYNTIC RECEPTOR Acetylcholine Stomach Wall Distention Cholinergic Pathways Acetylcholine Antral Distention H Protein in Meal SECRETION K H ATPase Long Vagovagal and Local Reflexes Local Reflex Gastrin Histamine PARIETAL CELL ANTRAL G CELL FIGURE 27-4 Neurochemical influences on gastric acid secretion. Bicarbonate also is secreted throughout the stomach and creates a pH gradient that neutralizes the hydrogen ions. A network of vascular capillaries beneath the surface epithelium provides yet another level of defense against gastric acid injury. Mucosal blood flow, through arterioles and capillaries, transports oxygen and substrates to the mucosa and removes acids that can be damaging to the epithelium of the stomach or duodenum.23 The rapid and continual renewal of gastroduodenal epithelial cells also enhances resistance to injury from secreted acids. In the majority of cases, disruption of the surface epithelium can be mitigated partially by the formation of a fibrin cap over the injured area (a process known as restitution).23,24 These actions of prostaglandin E, somatostatin, bicarbonate, gastric mucus, mucosal blood flow, epithelial cell regeneration, and restitution all combine to protect the gastric epithelium against injury from secreted acid. Pathogenesis The integrity of the upper GI mucosa depends on a balance between aggressive forces (primarily gastric acid and pepsin) and mucosal defensive factors.24 Gastric acid is necessary for the formation of ulcers, but an alteration in the protective defenses plays a significant role as well. Patients with ZE syndrome have an increased parietal cell mass, which secretes large amounts of gastric acid. Conversely, patients with duodenal ulcers have normal acid secretion, and patients with gastric ulcers tend to have normal or low acid production.24 These observations support the premise that other mechanisms are involved in the pathogenesis of ulcers. Studies have focused on alteration of mucosal defenses as an etiology of ulcers. For example, prostaglandin inhibitors (e.g., NSAIDs) may render the gastric mucosa susceptible to ulceration because endogenous prostaglandins protect the GI tract.26 H. pylori also has a role in the pathogenesis of PUD, and eradication of this organism can alter the natural history of the PUD. As a result, many investigators have divided PUD into three etiologic groups based on pathophysiologic abnormalities: (1) ulcers associated with H. pylori infection, (2) those caused by NSAID use, and (3) those with acid hypersecretion (i.e., ZE syndrome).24 Helicobacter pylori A Gram-negative spiral bacterium, Campylobacter pylori, was isolated in 1983 from patients with gastritis, and the association of this organism to the pathogenesis of PUD began to be investigated. Approximately 6 years later, the nomenclature of Campylobacter pylori was changed to H. pylori because the characteristics of this organism were more aligned with bacteria in the Helicobacter genus than the Campylobacter genus.25,26 Approximately 40% of patients older than 60 are seropositive for H. pylori, whereas the prevalence in patients younger than 30 years of age is only 10%.27 The mode of transmission of H. pylori is unknown; however, person-to-person transmission is likely because of findings of familial clustering.28 H. pylori can be isolated anywhere in the stomach but is found most consistently in the gastric antrum, where it is associated with inflammation. This organism is the most common cause of antral gastritis,29 and elimination from the gastric antrum is associated with resolution of gastritis. The exact mechanism by which H. pylori causes gastric injury is unknown, but possibilities include production of a cytotoxin, breakdown of mucosal defenses, and adherence to epithelial cells.30 H. pylori is unique in that it produces large amounts of urease. Urease catalyzes the hydrolysis of urea to form ammonia, and the accumulation of ammonia may play a role in disrupting the integrity of gastric mucosa, rendering it susceptible to ulceration.31 In addition, H. pylori infected individuals have increased basal and stimulated acid secretion when compared with those not infected. Eradication of H. pylori can reverse this increased acid secretion to normal.32 H. pylori is found in approximately 90% of patients with duodenal ulcers and in 70% of those with gastric ulcers.32 Eradication of H. pylori significantly decreases the recurrence rate of peptic ulcers and accelerates the ulcer healing process; therefore, H. pylori is implicated in the pathogenesis of PUD.33 Nevertheless, not everyone with H. pylori has an ulcer, and it is often isolated from patients who do not have PUD.33 H. pylori infection also is not associated with acute perforated duodenal ulcers. This latter finding suggests that perforated duodenal ulcers may have a different pathogenesis from chronic duodenal ulcer disease and that perforation is 27-6 • GASTROINTESTINAL DISORDERS not a complication of H. pylori infection.34 It is possible that NSAID use plays a significant role in this problem. Risk Factors A number of factors can predispose an individual to development of PUD: • Disruption of mucosal resistance to injury appears to be one mechanism involved in the pathogenesis of ulcers. The inflammatory response associated with H. pylori is thought to disrupt the architecture of the gastroduodenal mucosa, which then alters its natural defense mechanism. Antral gastritis associated with H. pylori infection has been found in 75% to 95% of patients with gastric and duodenal ulcers, respectively.24 • Overwhelming evidence associates NSAID use with PUD, particularly gastric ulcers. NSAIDs and aspirin impair ulcer healing and induce ulcer formation through prostaglandin inhibition and directly irritate the gastric and duodenal mucosa.36 • Cigarette smoking impairs ulcer healing, promotes ulcer recurrence, and increases the likelihood of ulcer complications.24 Cigarette smoking may cause ulcers through stimulation of gastric acid secretion and bile salt reflux,31,32 alteration in mucosal blood flow, and reduction in prostaglandin synthesis.4,32 • Historically, some foods were believed to be factors in the development of ulcers. Although some foods and beverages (e.g., caffeine-containing foods, milk, alcohol, spicy foods) increase acid secretion and cause dyspepsia, no scientific data support the belief that diet imparts an increased risk for ulcer formation.3 Acute alcohol ingestion can damage the gastric mucosal barrier, leading to acute gastric mucosal lesions and GI bleeding; however, alcohol has not been proven to cause PUD.4 • Genetic predilection appears to be a risk factor for the development of ulcers. Approximately 20% to 50% of patients with duodenal ulcers have a positive family history of PUD compared with 5% to 15% of nonulcer patients.20 • Although rigorous studies are lacking, stressful life events are thought by some to exacerbate PUD.32 Clinical Presentation Patients with gastric or duodenal ulcers present with similar symptoms and cannot be differentiated on the basis of clinical findings; therefore, a definitive diagnosis requires an upper GI radiologic series or an endoscopy is needed to visualize the ulcer. The most common and often the only symptom of an ulcer is epigastric pain. Overall, the pain associated with duodenal and gastric ulcers is not well localized and usually is described as annoying, burning, gnawing, and aching.3 Pain occurring when the stomach is empty (e.g., during the night, between meals) and when relieved by food and antacids is the most widely described characteristic of duodenal ulcer pain. Duodenal ulcer pain usually is episodic with symptomatic periods lasting for weeks followed by a period of no occurrence. The pain associated with gastric ulcers is difficult to distinguish from duodenal ulcers, but it may occur at any time of the day. Epigastric pain does not always correlate with the presence or absence of an ulcer; asymptomatic patients have been diagnosed with ulcers, and patients with dyspeptic symptoms may not have active ulcer disease.1,2 Gastric and duodenal ulceration can occur in the absence of dyspeptic symptoms and may be present in the elderly who are taking analgesics or in those who have a high pain tolerance. Complications such as bleeding, obstruction, or perforation often are often associated with a change in the character of a patient’s pain.2 Treatment The relief of symptoms, promotion of ulcer healing, prevention of ulcer recurrence and complications, and the provision of cost-effective therapy are among the primary treatment goals of uncomplicated PUD. It is especially important to remember that patients who present with PUD usually have a primary complaint of dyspepsia. Thus, relief of this symptom is the most important goal for PUD patient. Current therapy for acute uncomplicated ulcers is directed toward eliminating H. pylori, reducing gastric acidity, or enhancing mucosal defenses. When patients are infected with H. pylori, the eradication of this organism is the primary treatment goal because of its clear association with duodenal and gastric ulcers.35,36 Healing of and reducing recurrence of gastroduodenal lesions is associated with an effective eradication of H. pylori.36 In contrast, gastric ulcers typically are associated with normal or decreased acid secretion and are less frequently associated with H. pylori infection. This may be due to the association of gastric ulcers to NSAID use, a potential cause that is independent of H. pylori infection.35,36 Gastric ulcers can be associated with gastric carcinoma. This possibility must be addressed in the diagnosis. Drug treatment of peptic ulcers (Tables 27-1 and 27-2) generally consists of diagnosis and eradication of H. pylori as well as medications that neutralize gastric acid (e.g., antacids), inhibit acid secretion (e.g., H2-antagonists, and PPIs), and protect the gastroduodenal mucosa (e.g., sucralfate). Table 27-1 lists the antisecretory medications used, but it must be remembered that eradication of H. pylori is the primary treatment for PUD in infected individuals. Virtually all patients with a duodenal ulcer are infected. With the advent of antibiotic treatment for H. pylori, acid suppression alone is rarely indicated except for patients with bacteriologic cure and recurrent ulcers. H2-Antagonists MECHANISM OF ACTION H2-receptor antagonists inhibit the secretion of gastric acid. Histamine, released primarily from mast cells, binds to H2-receptors and activates adenylate cyclase, and thereby increases intracellular cyclic adenosine monophosphate (cAMP). The increased levels of cAMP activate the proton pump of the parietal cell to secrete hydrogen ions against a concentration gradient in exchange for potassium ions.37 H2receptor antagonists competitively and selectively inhibit the action of histamine on the H2-receptors of the parietal cells, thus reducing basal and stimulated gastric acid secretion. EFFICACY There are four H2-receptor antagonists available in the United States. All are effective for the treatment of duodenal and gastric ulcers (see Table 27-1). Use of these medications is associated with an overall healing rate of 70% to 95% after 4 to 8 weeks of therapy. They are equally effective in the acute healing of duodenal and gastric ulcers and are equally well tolerated.38 UPPER GASTROINTESTINAL DISORDERS Table 27-1 • 27-7 Antiulcer and GERD Indications H2RAs Cimetidine Famotidine Nizatidine Ranitidine Proton Pump Inhibitors Esomeprazole Omeprazole Lansoprazole Pantoprazole Rabeprazole Other Agents Misoprostol Sucralfate PUD Hypersecretory GERD Esophagitis Xa,b Xa,b Xa,b Xb,c X X X X X X X X X X Xa Xb,c X X X X X X X X X X X X X X Xc NSAID-Induced Ulcer X Xd,e Xe Xb,c a Duodenal and gastric ulcer. Maintenance for duodenal ulcer. c Duodenal ulcer. d NSAID-induced ulcer healing. e NSAID-induced ulcer prevention. X, approved indication. b Table 27-2 Antiulcer and GERD Medications: Doses Duodenal Ulcer H2RAs Cimetidine Famotidine Nizatidine Ranitidine Proton Pump Inhibitors Esomeprazole Lansoprazole Omeprazole Pantoprazole Rabeprazole Other Agents Sucralfate 300 mg QID 400 mg BID 800 mg HS 20 mg BID 40 mg HS 150 mg BID 150 mg BID 300 mg HS 30 mg QD 60 mg QD 20 mg QD 40 mg QD 20mg QD Duodenal Ulcer Maintenance Gastric Ulcer GERD Esophagitis Hypersecretory 400 mg HS 300 mg QID 800 mg BID 400 mg QID 800 mg BID 400 mg QID 300 mg QIDa 20 mg BID 40 mg HS 20 mg BID 20 mg BID 20 mg BIDa 150 mg BID 150 mg HS 150 mg BID 150 mg BID 150 mg BID 150 mg BID 150 mg QID 150 mg QID 150 mg BIDa 15 mg QD 30 mg QD 20 mg QD 30 mg QD 20 mg QD 30 mg QD 60 mg QDa 60 mg QDa 10 mg QD 20 mg QD 40 mg QD 20 mg QD 20 mg QD 60 mg QDa 40 mg QD 20 mg QD 40 mg QD 20 mg QD 40 mg QDa 60 mg QDa 20 mg QD NSAID 30 mg QDb 1 mg QID 2 gm BID Misoprostol 200 g QIDc a Titrate dose to acid secretory response. Prevention NSAID ulcers and NSAID ulcer healing. Prevent NSAID ulcers. BID, twice daily; HS, at bedtime; QD, once daily; QID, four times daily. b c POTENCY Although the relative antisecretory potency on a milligram-per-milligram basis of these four agents differs (famotidine has the greatest potency, followed by nizatidine, ranitidine, and lastly, cimetidine), this issue is not clinically relevant because the formulations of these four drugs have been adjusted accordingly. The serum concentration that is necessary to inhibit 50% of pentagastrin-stimulated secretion of acid determines the dosage and frequency of administration of each H2-receptor antagonist. Famotidine is the most potent and possess the longest duration of action.39 27-8 • GASTROINTESTINAL DISORDERS PHARMACOKINETICS Although these agents have identical mechanisms of action and similar clinical benefits, they differ in their pharmacokinetic profiles (Table 27-3). Oral absorption of all the H2-receptor antagonists is rapid, and peak drug concentrations usually are achieved within 1 to 3 hours after administration.39 The bioavailability is lower for cimetidine, famotidine, and ranitidine because they are absorbed incompletely and undergo first-pass hepatic metabolism. Ranitidine undergoes the most extensive first-pass metabolism, which accounts for the large difference between the oral and intravenous (IV) doses of this drug. Nizatidine is the only H2-receptor antagonist that is unavailable as an IV formulation. All four drugs are eliminated by a combination of hepatic metabolism, glomerular filtration, and renal tubular secretion.39 Hepatic metabolism is the principal pathway for the elimination of cimetidine and ranitidine, whereas renal excretion is the major route for the elimination of famotidine and nizatidine.39 For each of these agents, the half-life is increased, the total body clearance is decreased, and dosage reduction is recommended for patients with moderate to severe renal insufficiency. The pharmacokinetics of the H2-receptor antagonists appear to be unaffected by hepatic dysfunction; however, in patients with hepatic failure and renal insufficiency, dosage adjustment may be necessary. ADVERSE EFFECTS H2-receptor antagonists are remarkably safe and the frequency of severe adverse effects is low for all four drugs.40 The most common adverse effects include GI discomfort (e.g., diarrhea, constipation), CNS effects (e.g., mental confusion, headaches, dizziness, drowsiness), and dermatologic effects (e.g., rashes).40 Cimetidine has weak antiandrogenic effects, and its use in high doses has been associated with gynecomastia and impotence. Hepatotoxicity has occasionally been observed in patients taking H2-receptor antagonists. The patients who are at greatest risk for developing adverse Table 27-3 effects include the elderly, those taking higher doses, and those with altered renal function. DRUG INTERACTIONS Several drugs interact with the H2-receptor antagonists and, in particular, cimetidine (Table 27-4). Cimetidine binds to the cytochrome (CYP) P450 mixed-function oxidase enzyme system and inhibits the biotransformation of several drugs by the liver. It primarily affects oxidative drug metabolism while conjugation generally is not affected. The magnitude of cimetidine interaction with other drugs varies from patient to patient; however, it generally will reduce the clearance of another drug by approximately 20% to 30%.41 This interaction is most clinically significant with drugs that have narrow therapeutic ranges such as phenytoin, warfarin, and theophylline. Although ranitidine is more potent on a molar basis, it binds less intensely to the CYP P450 system than cimetidine.41 Therefore, when used in equipotent doses, there is less potential for ranitidine (compared with cimetidine) to interfere with the liver metabolism of other drugs.39 In contrast, famotidine and nizatidine do not bind appreciably to the CYP P450 system and therefore have limited ability to inhibit the metabolism of other drugs.41 Overall, ranitidine, famotidine, and nizatidine, when used in equipotent doses, are unlikely to cause clinically significant drug interactions when administered concurrently with other drugs eliminated by phase I oxidative metabolism by the liver. However, the introduction of cimetidine to a patient receiving a drug that requires the CYP P450 system for metabolism may require a downward dosage adjustment of the object drug to avoid increased serum concentrations. Patients receiving drugs known to interact with H2-receptor antagonists should be monitored to prevent or minimize the development of an adverse drug–drug interaction. Some of the H2-receptor antagonists are eliminated via renal tubular secretion and therefore have the potential to compete with cationic compounds for tubular secretion.42 Cimeti- Pharmacokinetic Comparison of H2-Receptor Antagonists Variable Cimetidine Ranitidine Nizatidine Famotidine Relative potency Absorption Bioavailability (%)b Time to peak serum concentration (hr) Volume of distribution (L/kg of body weight) Elimination Total systemic clearance (mL/min) Half-life in serum (hr) Hepatic clearance (%) Oral IV Renal clearance (%) Oral IV 1 4–10 4–10 20–50 30–80 (60) 1–2 0.8–1.2 30–88 (50) 1–3 1.2–1.9 75–100 (98) 1–3 1.2–1.6 37–45 (43) 1–3.5 1.1–1.4 450–650 1.5–2.3 568–709 1.6–2.4 667–850 1.1–1.6 417–483 2.5–4 60 25–40 73 30 22 25 50–80 25–30 40 50–80 27 50 57–65 75 25–30 65–80 a Adapted with permission from references 37–39. Average values are in parentheses. IV, intravenous. b UPPER GASTROINTESTINAL DISORDERS Table 27-4 • 27-9 Clinically Significant Drug Interactions With Cimetidinea Effect on Serum Drug Concentration Drug Phenytoin Nifedipine Procainamideb Theophyllineb Warfarin Benzodiazepines (diazepam, chlordiazepoxide) Carbamazepine Propranolol Quinidine ↑ ↑ ↑ ↑ ↑ ↑ ↑ ↑ ↑ TCAs (imipramine, desipramine, amitriptyline) Verapamil ↑ ↑ b Mechanism Inhibits metabolism; 140% ↑ serum levels ↑ AUC by 60–90% Competition for renal tubular secretion; 44% ↑ AUC Inhibits metabolism; 20–40% ↑ in serum levels Inhibits metabolism of R isomer; 20% ↑ PT Inhibition of metabolism; 30–60% Inhibition of metabolism; variable Inhibition of metabolism; 1.5- to 3-fold ↑ in plasma level Inhibition of metabolism and may ↓ renal clearance; 14.5% ↑ AUC Inhibition of metabolism; variable ↑ clearance by up to 35% a Adapted from references 40–45. There also are fewer interactions with ranitidine. AUC, area under the curve; PT, prothrombin time; TCAs, tricyclic antidepressants. b dine and ranitidine inhibit the renal tubular secretion of procainamide and its metabolite by this mechanism.43 Patients taking any one of these H2-receptor antagonists have a decreased area under the concentration-time curves (AUCs) of procainamide and N-acetyl procainamide and their renal clearance is decreased. The interaction with ranitidine appears to be concentration and dose dependent; therapeutic doses of ranitidine (i.e., 300 mg/day) did not cause an interaction, whereas large doses (i.e., 750 mg/day) alter the pharmacokinetics of procainamide.43 Although famotidine is a cationic drug that is excreted via active tubular secretion, it does not inhibit the renal elimination of procainamide or its metabolite.44 A possible reason for this discrepancy is that the serum concentrations attained by the usual therapeutic dose (i.e., 20 mg twice a day) of famotidine may be too low to compete with other drugs at the site of renal tubular secretion.39 All four H2-receptor antagonists can potentially affect the absorption and reduce the bioavailability of some drugs by altering the gastric pH.45 By increasing the pH of the GI tract, cimetidine slows the dissolution of ketoconazole, ultimately reducing its absorption. Most H2-receptor antagonists are weak inhibitors of gastric alcohol dehydrogenase. Thus, therapeutic doses of cimetidine, ranitidine, and nizatidine, enhance the absorption of ethanol following ingestion of moderate amounts of alcohol (approximately one or two glasses of wine or cans of beer).45 This interaction is thought to be a result of gastric alcohol dehydrogenase inhibition. According to these findings, systemic effects of alcohol may be exacerbated and the safety of patients compromised. Famotidine has little effect on this enzyme, but in a more recent study, H2-receptor antagonists did not alter serum ethanol levels following moderate to large alcohol consumption.46 Until more data are available confirming the results of the latter study, possible adverse effects associated with ethanol use should be considered when selecting an H2-receptor antagonist for patients who routinely consume alcohol. DOSING When cimetidine was first introduced, it was administered four times a day. However, based on clinical studies, recommendations now range anywhere from once daily to four times a day (see Table 27-2). The hypothesis that control of nighttime acid secretion is more effective in healing duodenal ulcers led to studies that compared night-time dosing with multiple-daily-dosing regimens finding similar healing rates.47 Proton Pump Inhibitors MECHANISM OF ACTION The proton pump inhibitors (PPIs) are highly specific inhibitors of gastric acid secretion. They act by irreversibly binding to K-H-ATPase (an enzyme that transports acid across the parietal cell), these drugs inhibit basal and stimulated gastric acid secretion in a dose-dependent and sustained fashion.48–50 PPIs cause almost total elimination of acid release because they inhibit the terminal step in the acid production cycle. EFFICACY The PPIs (i.e., omeprazole [Prilosec], lansoprazole [Prevacid], rabeprazole [Aciphex], pantoprazole [Protonix], and esomeprazole [Nexium]) relieve symptoms and heal duodenal and gastric ulcers more quickly than the H2-receptor antagonists (in 2 to 4 weeks compared with 4 to 8 weeks). However, the absolute healing rates for drugs in both groups are comparable (i.e., 90%) after completion of the requisite course of therapy.51 All the PPIs inhibit 90% of gastric acid secreted in 24 hours.52 While PPIs are indicated for the short-term treatment of active duodenal ulcers, eradication of H. pylori is the first-line treatment. PPIs are also effective in healing benign gastric ulcers, erosive reflux esophagitis, and controlling the hypersecretion associated with ZE syndrome. 27-10 • GASTROINTESTINAL DISORDERS PHARMACOKINETICS The PPIs are formulated as enteric-coated granules within capsules or enteric-coated tablets because they are unstable in acid media.53–55 Both omeprazole and pantoprazole are available in an IV formulation. Only IV pantoprazole is available in the United States. An IV formulation of lansoprazole is currently being investigated. All PPIs are rapidly absorbed after oral administration, with peak concentrations occurring 2 to 4 hours after administration of enteric-coated preparations.55 The bioavailability of these agents ranges from 50% to 80%. Currently available PPIs serve as prodrugs for the formation of active metabolites. The parent drugs are absorbed in the small intestine and brought to parietal cells via the systemic circulation. The active metabolite of omeprazole (omeprazole sulfonamide) and the active metabolites of lansoprazole (lansoprazole sulfone and hydroxy lansoprazole) are protonated in the acidic region of the gastric parietal cell secretory canaliculi. These active metabolites then bind covalently to sulfhydryl groups on the H-K-ATPase (proton pump) to noncompetitively and irreversibly inhibit this enzyme and block release of acid. The PPIs are eliminated almost entirely by hepatic metabolism, and the elimination plasma half-lives are approximately 1 to 2 hours.51–57 Despite the short plasma half-lives, antisecretory effect still is present 36 to 72 hours after a dose because the drugs bind covalently to the HK-ATPase in the parietal cell.55 Dosages need not be adjusted for patients with renal impairment, but adjustment may be prudent in those with severe liver disease.51–57 Esomeprazole, the S-enantiomer of omeprazole, has a similar pharmacokinetic profile to omeprazole. ADVERSE EFFECTS The adverse effects of these agents are relatively infrequent and comparable to those of H2-receptor antagonists. Some adverse effects include GI discomfort (e.g., nausea, diarrhea, abdominal pain), CNS effects (e.g., dizziness, headache), and isolated reactions (e.g., skin rash, gynecomastia, increase in liver transaminases).52 Theoretically, the PPIs can cause hypergastrinemia through their profound ability to inhibit gastric secretion because antral G-cells release gastrin when gastric pH increases.53 Chronic hypergastrinemia in humans is thought to lead to hyperplasia of enterochromaffin-like cells and carcinoid tumors of the stomach; however, there is no evidence of tumors actually occurring even after several years of continued treatment with high doses in patients with esophagitis.56 DRUG INTERACTIONS All PPIs bind to P450 oxidative enzymes and therefore potentially interfere with hepatic drug metabolism. Data indicate that omeprazole has a differential affinity for specific CYP P450 isoenzymes, especially CYP2C19, and can affect drugs primarily metabolized by that enzynme.58 Omeprazole decreases the metabolism of diazepam, phenytoin, warfarin, and tolbutamide and may increase the serum concentrations of these drugs. Patients taking these drugs with omeprazole should be monitored for any potential toxic effects. Although lansoprazole has not been shown to interact with drugs that are metabolized by the CYP P450 system, there are reports of slight decreases in the AUC for theophylline; this interaction may not be clinically insignificant.58 The bioavailability of omeprazole, lansoprazole, and probably the other PPIs may be reduced by sucralfate. Be- cause pantoprazole also is metabolized by a cytosolic sulfotransferase, it appears to interact less with drugs that compete with the P450 enzyme system. This may explain why pantoprazole may have a lower potential for drug interactions.58 Rabeprazole can modestly increase digoxin serum concentrations; however, no interactions have been observed with phenytoin, warfarin, or theophylline.5 Esomeprazole may inhibit the P450 enzyme system less than omeprazole.59 Sucralfate MECHANISM OF ACTION Sucralfate (Carafate) protects ulcerated tissue from aggressive factors such as pepsin, acid, and bile salts.60 At a pH of 2.0 to 2.5, sucralfate binds to damaged and ulcerated tissue, forming a physical barrier to injury from aggressive forces. The drug is not absorbed systemically and does not possess an antisecretory activity. However, sucralfate may have other protective actions on the mucosa that are possibly mediated by prostaglandin and gastric bicarbonate secretion. EFFICACY In doses of 1 g four times a day, sucralfate is more effective than placebo in healing duodenal ulcers61; it also appears to be as safe and effective as H2-receptor antagonists in the treatment of acute duodenal and gastric ulcers.62 The fourtimes-a-day dosing regimen for sucralfate is problematic for most patients, but studies indicate that a 2-g twice-daily regimen is as effective as 1 g four times daily in the short-term treatment of active duodenal ulcers.63 Sucralfate is effective, but not approved by the U.S. Food and Drug Administration (FDA), for acute or maintenance therapy of gastric ulcers. Although sucralfate is generally not used in H. pylori eradication regimens, one recent study demonstrated an eradication rate of 75% when used with amoxicillin and clarithromycin.64 ADVERSE EFFECTS Sucralfate is not appreciably absorbed systemically and therefore adverse effects are uncommon. The most common adverse side effect of sucralfate is constipation, possibly due to the aluminum content of the compound. Other side effects include dry mouth, nausea, and rashes. Sucralfate tablets are large, and some patients, particularly the elderly, may have difficulty swallowing them. Because sucralfate is available as both a tablet and a suspension, the liquid formulation can be used when patients encounter swallowing difficulties. DRUG INTERACTIONS The bioavailability of digoxin, fluoroquinolone antimicrobials, ketoconazole, levothyroxine, phenytoin, quinidine, tetracycline, theophylline, and warfarin may be reduced when concomitantly administered with sucralfate. The mechanism of the interaction is thought to be caused by binding of the agent with sucralfate in the GI tract. Because sucralfate may reduce the absorption of other drugs, it should be administered separately (e.g., 2 hours after other agents). Antacids MECHANISM OF ACTION Antacid products contain either sodium bicarbonate, aluminum hydroxide, magnesium hydroxide, calcium carbonate, aluminum phosphate, or a combination of these agents. UPPER GASTROINTESTINAL DISORDERS Antacids relieve epigastric pain and promote healing of peptic ulcers by providing a cytoprotective effect, neutralizing gastric acid, and stimulating restitution of the gastric mucosa.65 The cytoprotective effect of antacids may be related to the stimulation of prostaglandins involved in gastric mucosal defense. EFFICACY In clinical studies, antacids in various dosing regimens promoted the healing of duodenal ulcers in 46% to 88% of patients after 4 weeks of therapy compared with 24% to 45% of patients treated with placebo.65 Antacids need to be taken multiple times a day to neutralize gastric acid, and patient adherence to multiple-times-a-day regimens (e.g., 1 and 3 hours after meals and at bedtime) is difficult. As a result, antacids primarily are used to provide relief of ulcer pain and dyspepsia and are taken on an “asneeded” basis as often as the patient requires them. Antacids are thought to work within 5 to 15 minutes; however, the duration of relief is estimated to be only approximately 2 hours.65 Although antacids are available in both liquid and tablet formulations, the liquid formulation has a more rapid acid-neutralizing action than the tablets. Tablet formulations should be chewed well to maximize their action.65 Because of the inconvenient dosage regimen, the high incidence of adverse reactions (primarily diarrhea), and lack of coverage by most prescription plans, antacids are best used as a supplement to other PUD treatments for occasional dyspepsia. ADVERSE EFFECTS Sodium bicarbonate is a potent, rapidly acting antacid with a short duration of dyspeptic relief.65 It should not be used for prolonged periods because systemic alkalosis can result from the accumulation of bicarbonate. Aluminum hydroxide commonly is combined with magnesium hydroxide or another magnesium salt because, by itself, it has low acid-neutralizing capacity compared with magnesium hydroxide or calcium carbonate. Magnesium hydroxide usually produces diarrhea, but when combined with aluminum, it offsets aluminum’s constipating effects. Diarrhea usually predominates when large doses of aluminum- or magnesium-containing antacids are used. Small amounts of aluminum and magnesium are absorbed after antacid administration, and aluminum or magnesium levels can accumulate in patients with renal insufficiency. This is not a problem in patients with normal renal function because both are excreted effectively. Magnesium should be avoided in patients with significant renal impairment (creatinine clearance [ClCr] 20 mL/min) because accumulation can lead to CNS effects (e.g., sedation, nausea, vomiting). Calcium carbonate is a rapidly acting, very effective neutralizer of gastric acidity; however, high dosages of 4 to 8 g/day also have the potential to stimulate gastric acid production and to induce the milk-alkali syndrome (i.e., hypercalcemic nephropathy with alkalosis).62 DRUG INTERACTIONS Antacids, by alteration of gastric pH, can interfere with the absorption of drugs (e.g., digoxin, phenytoin, isoniazid) that require an acidic environment for dissolution and absorption.65 Ketoconazole, a dibasic compound, requires sufficient gastric acid for dissolution and absorption, and concurrent ad- • 27-11 ministration of ketoconazole with antacids can decrease the plasma concentration of this antifungal drug. When antacids increase gastric pH, they also have the potential to dissolve the enteric coating of concurrently administered medications, which are designed to dissolve in an environment more alkaline than the stomach, resulting in premature dissolution of enteric-coated drugs. By binding to concomitantly administered drugs, the calcium, aluminum, or magnesium component in antacids can interfere with the absorption of drugs that are susceptible to complexation with these salts. The bioavailability of ciprofloxacin is decreased by 50% by antacids because aluminum and magnesium ions chelate with the quinolone antibiotic to form and insoluble, inactive complex potentially leading to treatment failure. The administration of ciprofloxacin 2 hours before the antacid increases ciprofloxacin bioavailability more than when administered 2 hours after the antacid.66 Tetracycline is another antibiotic well known to interact with antacids. Antacids containing multivalent cations (Mg2, Ca2, Al3) have a strong affinity for tetracycline, and absorption of the antibiotic can vary according to the extent of complexation.66 Patients taking any form of tetracycline should be counseled not to take antacids for at least 2 to 3 hours after tetracycline administration. Overall, separating the administration times of each drug by 2 hours generally can minimize drug–drug interactions with antacids. Eradication of H. pylori We now know that most patients with a duodenal ulcer and many with gastric ulcers are colonized with H. pylori.36,67 Bismuth with acid suppression was initially used in the treatment of H. pylori–infected patients with peptic ulcers because it is active against H. pylori at a minimum inhibitory concentration of 16 mg/mL,67–69 and its use was associated with a reduction in ulcer recurrence.67,68 Patients who remain positive for H. pylori have a higher recurrence rate within the first year after healing compared with patients in whom eradication of H. pylori is achieved. A variety of regimens have been used to eradicate H. pylori, and most currently accepted regimens use several antibiotics plus acid suppression. MONOTHERAPY Although FDA-approved (omeprazole or lansoprazole plus clarithromycin) regimens are available, their eradication rates are in the 70% range. This is currently considered less than acceptable by experts.67 COMBINATION THERAPY Because monotherapy with antibiotics and monotherapy with acid suppressants has not been optimal, combination therapy consisting of antibiotics in conjunction with acid suppressants (PPIs or H2-receptor antagonists) have become the primary mainstays in the management of H. pylori–positive ulcer patients.71 In two prospective studies, H2-receptor antagonists were compared with combination antimicrobial therapy to evaluate the differential contributions of decreased acid secretion to H. pylori eradication and on the recurrence rate of ulcers.70,72,73 In these studies, recurrence rates for both gastric and duodenal ulcers after 1 year were significantly lower (approximately 10%) with combination therapy than with ranitidine therapy alone (approximately 85%).71,74 27-12 • GASTROINTESTINAL DISORDERS A variety of regimens have been used to eradicate H. pylori, each with different dosing schedules, durations of therapy, adverse effects, and costs. Several studies have evaluated antibiotic regimens for H. pylori eradication. Three were noted to be cost-effective and associated with good outcomes: (1) triple therapy of bismuth, metronidazole, and tetracycline for 14 days; (2) clarithromycin, PPI, and metronidazole or amoxicillin for 7 to 14 days; and (3) triple therapy of bismuth, metronidazole, and tetracycline, plus a PPI for 7 days.70–75 For patients who cannot receive metronidazole or when resistance to metronidazole is either established or suspected, the regimen of choice is 7 to 14 days of clarithromycin, amoxicillin, and a PPI. Another therapeutic regimen (i.e., bismuth 2 tablets four times a day with meals and at bedtime, metronidazole 500 mg four times a day, and amoxicillin or tetracycline 500 mg four times a day for 14 days) results in an eradication rate of 80% to 85%.74 RECOMMENDED COMBINATION REGIMENS Regardless of the regimen chosen, effective eradication of H. pylori requires combination therapy, and the ideal regimen contains two antibiotics combined with either a PPI or bismuth.67,75 Recommended H. pylori eradication regimens can be found in Table 27-5 and are discussed in further detail below. 1. PPIAC: The combination of a PPI, amoxicillin, and clarithromycin is approximately 90% to 95% effective and of moderate to high expense. Patient adherence to this regimen may be good because the regimen uses twice-daily dosing and can be for a 7- to 14-day duration. This regimen (using lansoprazole) is available commercially as Prevpac in a 14-day “bingo card.” When using this regimen, the PPI should be taken twice daily before meals for 14 days; the amoxicillin 1,000 mg twice daily should be taken with meals for 14 days; and the clarithromycin (Bi- Table 27-5 axin) 500 mg twice daily should be taken with meals for 14 days. More recently, 10-day PPI regimens with amoxicillin and clarithromycin have been approved by the FDA. Seven-day regimens are currently not FDA approved and are generally less effective than the 10- to 14-day regimens. All PPIs are equally effective and the choice can be determined based on cost and third-party prescription coverage. H2-antagonists should not be added to regimens that contain a PPI. 2. PPIMC: Metronidazole at a dose of 500 mg BID can be substituted for amoxicillin with similar rates of eradication.71,72 3. BMT-H2: The combination of bismuth, metronidazole, and tetracycline plus an H2-antagonist may be given generically as separate medications or in a carton with 14 daily blister cards and instructions (Helidac kit). This regimen is relatively inexpensive but is complicated, requiring four drugs administered four times daily for 2 weeks followed by an additional 16 days of H2-antagonist therapy. The bismuth is given as bismuth subsalicylate 262 mg (PeptoBismol) and the tablets (two) are to be chewed four times a day for 14 days with meals and at bedtime. Bismuth given on a four-times-a-day dosing schedule before meals appears to be more effective than twice-daily dosing.74 The metronidazole is taken as 250 mg four times a day for 14 days with meals and at bedtime. The tetracycline in this regimen is given as 500 mg four times a day for 14 days with meals and at bedtime. The effect of meals in decreasing the absorption of the tetracycline is not of concern because a local, rather than systemic, action is the objective. An H2-antagonist is needed for 30 days to enhance symptom relief and possibly enhance healing rate. A PPI taken twice daily can be substituted for the H2-antagonist. 4. RBC-C: The combination of ranitidine, bismuth citrate, and clarithromycin is available commercially as Tritec Helicobacter pylori Eradication Regimens Duration (days) Efficacy (%) PPIa BID Clarithromycin 500 mg BID Amoxicillin 1,000 mg BID 7, 10, 14 88–95 FDA approved (most PPIs); efficacy improves with increased duration of therapy; relatively simple regimen; available as “bingo card” (Prevpac) PPIMC PPIa BID Clarithromycin 500 mg BID Metronidazole 500 mg BID 7, 10, 14 88–95 Relatively simple regimen; efficacy improves with increased duration of therapy; caution with alcohol use BMT-H2 H2RAb Bismuth two tablets QID Tetracycline Metronidazole 14–28 75–85 Complicated regimen; FDA approved (“bingo card”); efficacy less than PPIAC or PPIMC RBC-C Ranitidine Bismuth subcitratec Clarithromycin 500 mg BID Ranitidine 150 mg BID 14 75–80 FDA approved Regimen Agents/Dose/Frequency PPIAC a PPI doses: omeprazole 20 mg BID; lansoprazole 30 mg BID; rabeprazole 20 mg BID; pantoprazole 40 mg BID; esomeprazole 20 mg BID. H2RA doses: cimetidine 400 mg BID; ranitidine 150 mg BID; famotidine 20 mg BID; nizatidine 150 mg BID. Ranitidine administered for 2 weeks after completing bismuth and antibiotics. b c Comment UPPER GASTROINTESTINAL DISORDERS (ranitidine 150 mg plus bismuth citrate 240 mg twice a day for 4 weeks combined with clarithromycin 500 mg three times a day for the first 2 weeks). This regimen is less effective (80% to 85% cure rates) and more costly. Moreover, the dosing schedule is complicated, the duration of therapy is longer (i.e., 4 weeks), and the regimen includes only one antibiotic. RBC may be an option in a penicillin allergic patient in whom it is desirable to avoid metronidazole due to ethanol use. 1. M.B., a 27-year-old female executive, presents with a 1week history of midepigastric upper abdominal pain, partially relieved both by eating and by calcium carbonate tablets (Tums), which she takes in the morning for calcium supplementation. She describes the pain as being moderately severe and different from gas pains which are associated with a bowel movement. She has not noticed darkening of the stool or the presence of blood in her stools. She has no other medical problems. In particular, she does not recall any chronic abdominal complaints such as gas, bloating, or heartburn and she has not been diagnosed previously with ulcer disease. She takes no other medications and is a nonsmoker. Her use of NSAIDs is limited to 200 to 400 mg of nonprescription ibuprofen tablets (Advil), but she does not take ibuprofen more than three times per week. What is the likelihood this patient has a peptic ulcer? Most patients with PUD initially present with nonlocalized epigastric pain as the only symptom, much like this patient. The relief of pain in this patient by both food and calcium carbonate makes one suspicious of a duodenal ulcer, but this in itself is not diagnostic. NSAIDs can increase the risk for the development of ulcer disease and she has been taking ibuprofen. However, her dose of ibuprofen is low, and she has used these drugs in the past without difficulty. Although a diagnosis of ulcer disease in this patient is uncertain based on her current history, she should discontinue the use of ibuprofen for now. 2. M.B. is counseled to use acetaminophen in place of ibuprofen and is given a prescription for famotidine (Pepcid) 40 mg HS to be taken for 6 weeks. What are reasonable courses of action for the management of this patient, and is famotidine treatment appropriate before a diagnosis of PUD has been established? If PUD is suspected, M.B. should be tested (serology or breath test) for H. pylori and if positive, receive an H. pylori eradication regimen. Endoscopic diagnosis before starting treatment is usually not indicated in cases of PUD for patients who are not suspected of having a complication. Endoscopy may be indicated if the patient is older than 55, if complications such as bleeding are present or suspected, if weight loss is present, or if epigastric symptoms have recurred after previous conservative treatment. It must be remembered that H. pylori testing alone does not diagnose the presence of an ulcer with certainty, because almost 50% of the population is colonized with this organism. A definitive workup (e.g., endoscopy, upper GI series) may be necessary if M.B. does not respond to the initial therapy, or if symptoms recur after initial therapy. Detection of Helicobacter 3. What available tests for the detection of H. pylori are appropriate for M.B.? • 27-13 H. pylori can be identified by direct testing of bacterial colonies from a culture medium or indirectly by biochemical assays and serologic tests. Because the organism is distributed unevenly throughout the gastric mucosa, at least two specimens from the antrum should be obtained.67,74,75 Histologic identification of H. pylori correlates well with culture results, with a sensitivity and specificity of 85% to 100% and 50% to 100%, respectively.67,74,75 Factors implicated in the failure to culture the organism include ingestion of simethicone, contamination of biopsy forceps with an antibacterial agent, recent treatment with antibiotics or PPIs, and biopsy in areas with low organism counts.67 The rapid urease test can detect the large amounts of urease produced by H. pylori within 2 to 3 hours with an endoscopically obtained biopsy specimen.67 In clinical practice, direct biopsy and culture are reserved for patients in whom other tests are nondiagnostic. Noninvasive tests for H. pylori are preferred when screening for this organism.67 The noninvasive breath test detects urease in the stomach subsequent to the oral administration of 13 C- or 14C-labeled urea. If urease is present in the stomach (presumably from H. pylori), the carbon dioxide exhaled in the breath will contain the 13C isotope (detected by mass spectrometer) and the 14C isotope (detected by a scintillation counter).67 The Meretek UBT (urea breath test) appears to be highly specific, but is costly and associated with 5% to 10% false-negative results.67 Another noninvasive test, the enzymelinked immunosorbent assay (ELISA) detects IgG and IgA antibodies to H. pylori in the serum. Sensitivity and specificity of these antibody tests are approximately 95%; however, a positive test can represent either past or present exposure to H. pylori. Serologic antibody tests may be helpful for the initial diagnosis of H. pylori but are more problematic for documenting eradication of this organism.67 However, long-term serologic surveillance investigations indicate that IgG and IgA anti–H. pylori antibody concentrations decrease rapidly and progressively after the eradication of the infection and increase with recurrence of H. pylori infection. As a result, these noninvasive serologic tests are used commonly to determine the presence of H. pylori because of their simplicity and relative low costs.68 Serology testing is now commonly used because of its simplicity and relative low cost. Breath testing is more sensitive, but cost prohibitive in many situations. Direct biopsy, culture, and the rapid urease tests require endoscopy and are reserved for situations in which endoscopy is warranted. In patients with recurrent or refractory ulcers, further testing should be considered because false-negative results are sometimes encountered.77,78 In this latter situation, other etiologies of PUD also should be evaluated. Although any of the aforementioned tests are acceptable for M.B., the most rational approach is testing with serology and eradication of H. pylori using one of the methods listed above. Treatment without testing for H. pylori has been advocated in some patients with a duodenal ulcer due to the high prevalence of H. pylori in patients with duodenal ulcers. However, since most duodenal ulcers are diagnosed by endoscopy, it is simple to biopsy the gastric mucosa and test for H. pylori. 4. Should M.B. discontinue using Tums tablets? Although there is no logic for taking full-dose scheduled antacids in conjunction with PUD treatment, patients generally are instructed to continue to take antacids on an as-needed 27-14 • GASTROINTESTINAL DISORDERS basis for breakthrough symptoms, especially for the first few days after starting treatment. In this particular situation, M.B. has been taking the Tums as a calcium supplement for osteoporosis prevention. Although she is still at low risk for osteoporosis because of her young age, there is no need to discontinue the Tums. She should be instructed to take extra doses as needed and to contact her physician or pharmacist if symptoms have not resolved within 2 weeks. 5. PPIs are superior acid suppressants compared with H2-antagonists. Would it be better to initiate therapy in this patient with a PPI? The PPIs are superior to the H2-antagonists in acid suppression and provide more rapid symptom relief. Despite this, acute treatment of patients with PUD by either H2-antagonists or PPIs without H. pylori eradication is not indicated. There is probably little, if any, role for PPIs or H2-antagonists for acute treatment of peptic ulcers except as part of an H. pylori regimen. This is due to the rapid recurrence of the ulcer after completing an acute regimen. 6. J.L., a 37-year-old woman, is in the clinic today for a routine follow-up after treatment for a second episode of suspected peptic ulcer. She relates a 14-year history of multiple GI complaints including postprandial bloating, “fullness,” belching, occasional nausea, reflux, and heartburn. There is no predictable pattern to these complaints; they may be present daily for weeks at a time, while at other times they may occur just a few times per week. The heartburn and reflux may occur in the absence of the bloating, belching, fullness, and nausea and vice versa. She has taken a variety of antacids over the years including Maalox, Mylanta, generic equivalents of both, Gaviscon, and Tums, all of which give partial or complete, but temporary, relief. Four years ago, J.L. had one episode of increased mid-upper epigastric pain that lasted for several days. The discomfort was relieved by food and was present during the night. Bowel movements had no discernible effect on the pain. Her physician informed her that she probably had an ulcer and prescribed a 10week regimen of cimetidine 400 mg twice a day. The abdominal pain abated, but since then she has continued to take either cimetidine or nonprescription H2-antagonists as needed almost daily for her chronic symptoms. Six weeks ago, a similar abdominal pain developed and she was treated empirically with clarithromycin 500 mg three times daily for 2 weeks and omeprazole 20 mg twice a day for the first 2 weeks and then 20 mg of omeprazole daily for an additional 2 weeks. Once again, the acute pain subsided, but her underlying complaints remain. She states that she was compliant most of the time, but sometimes had difficulty remembering all three doses of the clarithromycin each day. However, she continued to take them until they were all gone. She lost track of how long it took for all of the medicine to be gone. The only other medication she has taken in the past year is an oral contraceptive, Triphasil (ethinylestradiol/levonorgestrel), which she has been taking for many years. She takes acetaminophen for headaches and avoids NSAIDs. She also relates multiple episodes of bladder/urethral and vaginal infections over the past 16 years, treated variously with trimethoprim-sulfamethoxazole, clotrimazole, and metronidazole. The last time she had any of these infections was 5 years ago. She has no other medical problems despite a 15-year history of cigarette smoking. She has never undergone endoscopy. H. py- lori testing was never performed before any of her treatments, but an H. pylori serology taken 5 days ago is positive. No other laboratory test results are available. Based on the data provided, is this patient’s history consistent with PUD? If endoscopy were to be performed today, is it likely that an active ulcer would be found? In the absence of endoscopy, it is impossible to know if this patient has ever had an ulcer. However, the following scenario could be hypothesized. For 14 years, she either experienced nonulcer dyspepsia, GERD, and/or PUD. The postprandial bloating, fullness, and nausea are all consistent with nonulcer dyspepsia. The reflux and heartburn could be either true GERD or a variant of nonulcer dyspepsia. In addition, PUD can also present with these symptoms. Thus, the acute change of symptoms 4 years ago, with the resolution after a course of H2-antagonists, is consistent with an acute ulcer followed by healing. However, her symptoms continued. Six weeks ago, it appeared as though she once again developed a peptic ulcer. This is not surprising considering her failure to receive H. pylori eradication treatments. During this second presumed ulcer episode, her primary care provider decided to try H. pylori eradication with a combination of a single antibiotic and a PPI. Once again the acute symptoms resolved. Thus, one might conclude that this patient does indeed have a history of PUD, but endoscopy is needed for further diagnosis. 7. Does the presence of a positive H. pylori serology 5 days ago indicate treatment failure of the antibiotic–PPI regimen? One should not conclude that the positive H. pylori serology in this patient indicates treatment failure. It should be recalled that H. pylori serology is the simplest and least expensive way to test for H. pylori infection. However, this test is limited because it only indicates the presence of antibodies to H. pylori, not the actual organism itself. Thus, it remains positive both in persons with an active infection and in those after successful eradication of the organism. Serology testing is most useful as a screening tool in an individual who has never received antibiotics that are active against H. pylori. In that situation, a positive test presumably is a marker of an active infection, although false-positive results may still occur. Although some data indicate that antibody titer levels decline after successful eradication, this is a less sensitive measurement tool than an enzymatic breath test that indirectly indicates the presence of living organisms, or results from a rapid urease test of endoscopically derived tissue. Furthermore, none of the confirmatory test procedures should be done within 4 to 6 weeks of the last dose of a PPI because these drugs suppress H. pylori growth without actually killing the organisms. In this patient, we have no prior antibody titers for comparison. The only conclusion that can be drawn from her positive serology is that she has been infected with H. pylori at some time in her life. She may or may not be infected now. If she had tested negative for H. pylori antibodies, the clinician would have suspected either a false-negative result from her recent omeprazole treatment, an ulcer not associated with H. pylori (which is relatively uncommon), or the absence of PUD entirely. 8. Because of the lack of specificity of the H. pylori serology test, a carbon isotope urea breath test is ordered. This test indicates a continued infection. What factors may have contributed to the lack of eradication of H. pylori in this patient? UPPER GASTROINTESTINAL DISORDERS Although the failure of medications to eradicate the H. pylori in this patient can be attributed to several variables, the issue of J.L.’s adherence to her prescribed regimen should be considered. However, she has stated that she did complete the regimen despite having missed some doses. One other explanation could perhaps be focused on a poor choice of antibiotic regimen (i.e., only one antibiotic with a PPI). • 27-15 doscopy is undertaken and if the endoscopic sample is positive for H. pylori, treatment is indicated because of the association between H. pylori infection and gastric cancer. The treatment options for GERD are presented in later cases. Drug-Induced Peptic Ulcer Disease Etiology and Clinical Presentation 9. Should patient J.L. be re-treated at this time? If so, what antibiotic regimen would presumably be the most appropriate? Although J.L. no longer has acute epigastric pain right now, she is at high risk for a recurrence of peptic ulcers if she still is infected with H. pylori. The most successful regimens for eradication of H. pylori contain either a PPI or a bismuthcontaining compound (generally bismuth subsalicylate in the United States) plus two of the following antibiotics as described above. It is known that this patient had difficulty complying with her previous regimen, which was relatively simple. Thus, she may not do well with the BMT regimen of bismuth (Pepto-Bismol), metronidazole, and tetracycline, which requires a total of 16 tablets and capsules four times daily for 14 days plus an H2-antagonist or PPI for 2 to 4 weeks. In reality, the risk of resistance may be remote because it has been several years since she took the metronidazole. Using the Helidac kit, in which the medications are supplied in a carton with 14 daily blister cards and instructions for use, could enhance compliance. A better regimen might be a PPI plus amoxicillin (or metronidazole) and clarithromycin for 10–14 days. The shorter course may enhance compliance. The most important principle is to develop a regimen with a high likelihood of success. Although cost of the medications is often foremost in the minds of third-party payers, this should not be a primary consideration because successful treatment will save much more in future costs than re-treatment, physician’s visits, and hospitalization for complications. The most expensive regimen is the one that the patient does not take or that does not work. 10. What is the likelihood that the urea breath test could have been reported back as a false-negative result? How would a negative test result alter the treatment plan for this patient? The single antibiotic regimen, which was chosen for this patient, is not optimal. The combination of A PPI plus amoxicillin will result in bacteriologic cure in 40% to 50% of cases. If the H. pylori in this patient has been eradicated, no further antibiotic treatment is necessary. A false-negative urea breath test is also possible, especially if the patient is tested too soon after the initial treatment. Nevertheless, further workup (e.g., retesting or endoscopy) is not required unless symptoms recur. As many as 40% of patients who have had successful H. pylori eradication will continue to require acid suppression medications to control persistent dyspeptic symptoms. Thus, clinicians should remember that bacteriologic cure might not provide complete relief of epigastric symptoms because patients also may have concurrent nonulcer dyspepsia or GERD. In this particular case, it should be recalled that this patient still has symptoms and that these may be suggestive of nonulcer dyspepsia or GERD. Dietary adjustments, simethicone, and/or H2-antagonists may or may not help provide symptom relief for J.L.’s nonulcer dyspepsia. If GERD is suspected, endoscopy may be necessary to rule out esophagitis. If en- 11. A.C., a 65-year-old woman, experienced orthostasis and diarrhea after attending a lecture. She has been in good health without any previous complaints. She has not been taking any medications other than ibuprofen 400 mg Q 4 to 6 hr PRN for headaches and arthritic knees for the past 4 weeks. Laboratory tests are unremarkable except for a hemoglobin (Hgb) of 8 g/dL, a hematocrit (Hct) of 26%, and a guaiac-positive stool. A.C. was admitted to the hospital for an endoscopy to rule out bleeding from the GI tract. Endoscopy revealed two antral ulcers (0.2 cm and 0.4 cm). What is the cause of A.C.’s GI bleeding? [SI units: Hgb, 4.96 mmol/L; Hct, 0.26] A.C.’s GI bleeding probably is most likely secondary to chronic ingestion of ibuprofen, which is an NSAID. The prevalence of endoscopically confirmed GI ulcers in NSAID users is 15% to 30%.80 Although most NSAID-induced ulcers are gastric ulcers (12% to 30%), duodenal ulcers (2% to 19%) are observed as well.81,82 Some investigators hypothesize that duodenal ulcers associated with NSAID use may represent an exacerbation, reactivation, or complication of PUD, because there is a higher incidence of duodenal ulcers unrelated to NSAID use.80 12. Why did A.C. not experience epigastric pain symptoms associated with NSAID-induced ulcers? Symptomatic ulcers (ulcers associated with pain, bleeding, or perforation) occur in only approximately 1% of patients after 3 to 6 months of NSAID use and in 2% to 4% of patients after 1 year of therapy.69 NSAID-associated ulcers do not correlate well with pain possibly because the analgesic action of NSAIDs may mask the ulcer pain.80 As illustrated by A.C., patients often take NSAIDs for chronic pain conditions such as arthritis; therefore, their perception of pain may be changed and they may tend to ignore modest epigastric pain. The reason is not clear, but NSAID ulcers in many patients are asymptomatic. Thus, complications (e.g., GI bleeding, syncope) may develop in the absence of typical symptoms. It is not unusual to find perforations, bleeding, or gastroduodenal ulcerations in asymptomatic individuals.69 The orthostasis experienced by A.C. probably is merely reflective of volume loss secondary to GI bleeding, and epigastric pain simply was not present or was masked. Mechanism of Action 13. How do NSAIDs cause ulcers? The most common site for NSAID-associated ulcers is the antral portion of the stomach, although some occur in the gastric body and fundus.69 NSAIDs appear to produce gastric damage by two mechanisms: a direct irritant effect and a systemic effect mediated by cyclo-oxygenase (COX) inhibition leading to a reduction in prostaglandin synthesis.76,81,82 Therefore, both rectal and IV administration of NSAIDs can cause 27-16 • GASTROINTESTINAL DISORDERS GI damage despite the apparent absence of direct mucosal contact.81 Agents that are more water soluble (e.g., aspirin) cause more topical injury.82 Aspirin, in a dose-related fashion, disrupts the gastric mucosal barrier. When the consumption of aspirin exceeds 22 tablets a week, the occurrence of ulcers is increased.76 Although enteric-coated tablets are designed to minimize gastric irritation, the potential for ulceration still exists but to a lesser degree.80 Inhibition of mucosal prostaglandin synthesis is thought to be an important factor in the pathogenesis of aspirin- and NSAID-induced ulcers.80,83 COX-1 is responsible for producing prostaglandins that protect the GI mucosa by maintaining blood flow and stimulating bicarbonate and mucus production.76 Therefore, low levels of prostaglandin would be expected to impair the ability of the gastric mucosa to defend itself against aggressive factors. An isoform of COX-1, COX-2, is involved in the inflammatory response. Both isoforms have the same affinity to convert arachidonic acid to prostaglandin; however, the prostaglandins produced by COX-2 are associated with pain and inflammation. COX-2 inhibition is believed to be responsible for the anti-inflammatory and analgesic effects of NSAIDs, whereas inhibition of COX-1 may be associated with NSAID adverse effects including GI and renal effects.80,83 Therefore, NSAIDs such as celecoxib (Celebrex), rofecoxib (Vioxx), and valdecoxib, which have the greatest inhibitory effects on COX-2 while maintaining minimal or no inhibition on COX1, will have the maximum effect on inflammation and minimal (but not totally eliminated) mucosal-damaging adverse effects.69 Treatment 14. What therapeutic steps should be taken in the treatment of A.C.’s gastric ulcer? When peptic ulcers develop in patients taking NSAIDs, the preferred approach is to stop the NSAID when possible. Ulcers heal slowly during treatment with H2-antagonists when NSAIDs are continued and more quickly when they are stopped.69 Therefore, A.C. should discontinue or decrease the dose of the ibuprofen and she should be evaluated for H. pylori infection. For mild to moderate pain, acetaminophen or another analgesic option can be used. If an anti-inflammatory action is required for her arthritic knee, a COX-2 NSAID can be used.69 Variables that may influence the healing of NSAIDassociated ulcers include continued NSAID therapy, ulcer site, ulcer size, history of PUD, and the presence of H. pylori gastritis.69,84–88 Discontinuation of the NSAID may allow for mucosal restitution and more rapid ulcer healing. The continued use of NSAIDs does not appear to prevent healing of small ulcers; however, the healing process may be delayed. H2-antagonists are generally not effective in preventing or healing NSAID-associated gastric ulcers when the NSAID is continued. An exception is famotidine given at a dose of 40 mg twice daily.69,87 Double doses of other H2-antagonists have not been studied. Another option is to test for and treat for H. pylori (if positive). If H. pylori testing is negative, a PPI may provide a more rapid resolution of symptoms and quicker healing of the ulcer. If the COX-2 is not an effective alternative for A.C. and if she must continue with her NSAID for management of her arthritis, a PPI would be preferred over an H2-antagonist. The H2-antagonists are not very effective for healing or preventing NSAID-associated gastric ulcers during continued therapy with NSAIDs.69 15. A.C.’s ulcer has healed after 8 weeks of omeprazole 20 mg/day and the discontinuation of her NSAID. However, her arthritic knees continue to be a problem, and NSAID therapy needs to be reinstated. What concurrent medications should A.C. receive to minimize future ulcer formation? Prophylactic therapy or a COX-2 NSAID should be considered for patients who are unable to discontinue NSAID therapy and who have risk factors for NSAID-associated ulcers. Patients particularly at risk include those who are taking concurrent anticoagulants or corticosteroids; those with a history of ulcer complications or ulcer disease; those who are elderly; and high-surgical risk, debilitated patients who cannot tolerate complications.69 If a COX-2 NSAID is chosen, the clinician must remember that these agents do not provide complete freedom from ulceration and other GI-associated complications of NSAIDs. H2-RECEPTOR ANTAGONISTS A few studies have examined prophylactic regimens for NSAID-associated ulcers. The H2-receptor antagonist, ranitidine, has been associated with a decrease in the incidence of NSAID/aspirin-associated duodenal ulceration, but this association was not apparent in patients with gastric ulcers.69,81,86 Double dose famotidine (40 mg twice daily) in another study was noted to have reduced the incidence of both gastric and duodenal ulcers compared with placebo in patients receiving long-term NSAID therapy.87 In this study, famotidine at either 20 mg or 40 mg twice a day was more effective in preventing duodenal than gastric ulcers. Some investigators postulate that NSAID-associated duodenal ulcers represent an exacerbation of pre-existing duodenal ulcer disease.69,87 The H2antagonists seem to be more effective in the prevention of NSAID-induced duodenal ulcer disease than in the prevention of NSAID-induced gastric ulcers. Misoprostol (Cytotec), a synthetic prostaglandin E1 analog, has both antisecretory and cytoprotective properties and can prevent both duodenal and gastric ulcers in the NSAID/ aspirin user.88–90 Despite the effectiveness of misoprostol in the prevention of duodenal and gastric NSAID-associated ulcers, it paradoxically causes GI symptoms such as diarrhea and abdominal pain.69 Diarrhea is dose dependent and is due to stimulation of intestinal smooth muscle and fluid and electrolyte secretion. Lowering the dose to 100 g four times a day or giving the dose less frequently (i.e., 200 g twice daily) decreases the incidence of diarrhea, but this dose also is less effective in preventing ulcers.69,89 Misoprostol is contraindicated in women of child-bearing age unless adequate contraceptive measures are taken, because prostaglandins are uterotonic and capable of increasing the frequency of uterine contractions, bleeding, and abortions.90 PROTON PUMP INHIBITORS In one study, the efficacy of omeprazole was compared with misoprostol in 935 patients who required continuous NSAID therapy and who had gastric and/or duodenal ulcers. UPPER GASTROINTESTINAL DISORDERS At 8 weeks, treatment was successful in 76% of the patients given omeprazole 20 mg daily, in 75% of those given omeprazole 40 mg daily, and in 71% of those given misoprostol 200 g four times a day.91 The rates of gastric ulcer healing were significantly higher with omeprazole 20 mg daily (87%) than with misoprostol (73%). Healing rates among patients with duodenal ulcers were higher (85%) with omeprazole 20 mg daily than with misoprostol (74%), whereas healing rates among patients with erosions alone were higher with misoprostol (84% versus 77%). More patients remained in remission during maintenance treatment with omeprazole (61%) than with misoprostol (48%) and with either drug than with placebo. More adverse events were noted during the healing phase in the misoprostol group than in the groups given omeprazole.91 The success of ranitidine, misoprostol, and omeprazole in preventing NSAID-associated duodenal ulcers suggests that acid suppression is important in the prevention of duodenal ulcers. A.C. is a candidate for prophylactic therapy because she is elderly, requires continued NSAID therapy, and has an ulcer history. The PPIs appear to be more effective in the healing of gastric ulcers than misoprostol and more effective than the H2-antagonists in healing and preventing ulcers.69 Therefore, it would be reasonable for A.C. to receive omeprazole 20 mg daily to minimize the risk of ulcer recurrence that is associated with NSAIDs. A COX-2 NSAID may also be an option. ZOLLINGER-ELLISON SYNDROME Pathogenesis ZE syndrome is a rare (occurring in fewer than 1 in 10,000 patients with DU) condition characterized by a triad of clinical findings, which include severe recurrent PUD, significant hypersecretion of gastric acid, and non–-islet tumors (gastrinomas) of the pancreas.92 Normally, G-cells in the antral mucosa secrete gastrin, which stimulates the parietal cells to secrete gastric acid. In ZE syndrome, an ectopic source (gastrinoma) also secretes gastrin with a subsequent hyperstimulation of gastric acid secretion.92 Although gastrinomas are located primarily in the pancreas, they also have been found in other regions, particularly the duodenum. Clinical Presentation 16. M.J., a 62-year-old man, was referred from his private physician for evaluation of his continuing ulcers. He has been treated for 2 to 3 years for PUD and has not experienced relief despite H. pylori suppression and escalating doses of various H2receptor antagonists. M.J. was evaluated twice to rule out a possible myocardial infarction because of severe chest pain. He also complains of frequent diarrhea and abdominal pain. All routine laboratory tests were found to be within normal limits except a serum gastrin level, which was 5,243 pg/mL (normal, 0 to 100 pg/mL). Radiographic and endoscopic studies demonstrate a distal esophageal ulceration, dilation of the duodenum and jejunum with thickened folds, and a 0.1-cm ulceration in the distal duodenum. After a secretin test, his serum gastrin level was 400 pg/mL greater than the basal level. M.J. was diagnosed with ZE syndrome. Why is M.J.’s presentation consistent with ZE syndrome? [SI unit: gastrin, 5,243 ng/L] • 27-17 Diagnosis The diagnosis of ZE syndrome is based on gastric acid hypersecretion (basal acid output 15 mmol/hr in a patient without prior gastric surgery or 5 mmol/hr in a patient with prior acid-reducing surgery) in conjunction with a fasting serum gastrin level 1,000 pg/mL.93,94 Some healthy individuals and patients with duodenal ulcers may have marginally increased serum concentrations of gastrin.93 Therefore, a provocative test is often used to identify patients with ZE syndrome. The secretin provocative test is the most sensitive, reliable, and simplest test for diagnosing and predicting the probability of a patient to remain disease free.93 If a patient has ZE syndrome, IV secretin will cause a significant increase in serum gastrin.93 A positive test, consistent with the diagnosis of gastrinoma, is defined as an absolute increase in serum gastrin of 200 pg/mL from the basal concentration.94 M.J.’s baseline and stimulated serum gastric levels are well above normal. Symptoms of ZE syndrome are similar to severe PUD but may be more persistent and less responsive to standard antiulcer therapy.93–95 This is consistent with M.J.’s 2- to 3-year history of unrelenting pain despite H. pylori eradication and high doses of H2-receptor antagonists. Many patients have no endoscopically or radiographically detectable GI ulcers at the time of diagnosis (18% to 25%), whereas others present with solitary or multiple ulcers (as in M.J.’s case).95 Most patients with ZE syndrome have an ulcer, usually in the duodenum or jejunum. Because the disease often presents as a routine peptic ulcer, the diagnosis of ZE syndrome may be delayed for a prolonged time.95 M.J.’s history is consistent with this description because he was evaluated in the past for complaints resembling a severe peptic ulcer, but ZE syndrome was not considered until radiographic evidence was present. Abdominal pain and diarrhea (both present in M.J.) are the most common symptoms at presentation and may occur in the absence of a mucosal ulcer.95 Diarrhea occurs in approximately 30% to 50% of patients and is caused by increased amounts of gastric acid. High gastric acid levels inhibit sodium and water absorption in the jejunum and damage the GI mucosa. Abdominal pain remains the most common symptom of ZE syndrome and is localized or diffuse in nature. The pain is similar to that found in a duodenal ulcer in that food and antacids often relieve it. Treatment 17. A PPI has been prescribed for M.J. Why would PPIs be more likely to be successful in the management of ZE compared with H2-antagonists? Because of the excessive amounts of gastric acid produced by patients with ZE syndrome, patients have an increased chance of severe GI discomfort, bleeding, and gastroduodenal perforation. Before the routine use of acid secretory suppressant medications (H2RAs and PPIs), total gastrectomy was the treatment of choice. Now, most patients can be effectively treated using PPIs. Despite this, some patients will require a surgical excision of the gastrinoma to control symptoms associated with excessive gastric acid production that persist despite large doses of PPIs.95 Most patients will be adequately managed with medical treatment which usually provides symptomatic pain relief and control of gastric acid secretion. 27-18 • GASTROINTESTINAL DISORDERS An acceptable criterion for long-term control of gastric secretion is a postdrug gastric acid secretion of 10 mEq/hr for the last hour before the next dose of medication.95 Successful medical management requires individualized titration of the drug dose with periodic reassessment. Histamine2-antagonists were the first drugs successfully used to control gastric hypersecretion, but they are not totally effective in the medical management of ZE syndrome. Large doses and more frequent dosing than typically used to treat PUD are generally required (see Table 27-2).95 This is due to the very high doses needed are usually inadequate to control the high gastric acid secretion.79 It is likely that total acid suppression can never be achieved by H2-antagonists alone because these drugs do not directly suppress either gastrin or acetylcholine. As a result, PPIs have become the treatment of choice for ZE. PPIs have an advantage over H2-antagonists because by selectively and irreversibly binding to H-K-ATPase, they can inhibit nearly all gastric acid production irrespective of the cause. In addition, PPIs have a long duration of action and thus require less frequent dosing than the H2-antagonists. All PPIs are effective for ZE syndrome, large doses generally are necessary, and doses should be adjusted as needed.77 Although surgical correction of the gastrinoma is likely, M.J. should begin at least 60 mg/day of omeprazole and titrated to response. Patients frequently are seriously ill when the initial diagnosis of ZE syndrome is confirmed, and it is important that the high gastric acid secretion be controlled.95 The total daily dose should be given in two to three divided doses initially.78,95 Octreotide, is a potent inhibitor of gastrin and an option in the management of ZE; however, because it is only available as a parenteral product, its use is limited.78 Proton Pump Inhibitor Administration 18. M.J. is scheduled for surgery, and omeprazole is to be administered through an nasogastric (NG) tube. What difficulties are likely to be encountered by this order, and what are reasonable solutions? In patients with NG tubes who are unable to take medications by mouth, drugs are often placed either directly into the NG tube or crushed and mixed with water to make a slurry/ suspension. Omeprazole, lansoprazole, and esomeprazole are formulated as enteric-coated granules within gelatin capsules because they are unstable in acid media and must be absorbed to become converted to their active metabolites. Analogous to sustained-release products, the enteric-coated beads should not be chewed or crushed because they are designed to dissolve in the GI tract when the pH of the medium is 6.95 However, when the capsules are opened, added to a bicarbonate solution, and the enteric-coated beads are not crushed, the AUC and the effectiveness of gastric acid suppression of enteric-coated omeprazole granules delivered via an NG tube can be comparable to that of encapsulated enteric-coated granules taken orally.98 Esomeprazole has been studied by opening the capsule and suspending in water.99 Suspensions of omeprazole and lansoprazole compounded from capsules and sodium bicarbonate solution are stable for up to 14 days at 24°C.100,101 When the lansoprazole gelatin capsule is opened, the intact granules can be mixed in 40 mL of apple juice and adminis- tered in a suspension of granules via the NG tube according to the manufacturer’s instructions. While FDA approved, this method is difficult to administer properly, as the granules sink to the bottom of the syringe used to inject into the NG tube. This apple juice and lansoprazole granule suspension must be agitated in the syringe as it is injected into the NG tube. Failure to do this, will administer a “bolus” of granules into the NG tube and will likely clog the tube. Crushing pantoprazole tablets and preparing a suspension in sodium bicarbonate has also been studied and found to produce blood levels similar to intact tablet administration.102 Continuous IV H2-receptor antagonists can be used to control gastric acid hypersecretion adequately in patients with ZE syndrome during surgery.103 Frequent dosage adjustments are required may be required in the perioperative period to control gastric acid output. IV omeprazole has been available in Europe for many years. As PPIs also are more effective in controlling gastric hypersecretion, IV pantoprazole may be the most effective option for M.J. Other IV formulations of PPIs are being developed GASTROESOPHAGEAL REFLUX DISEASE Gastroesophageal reflux is a disorder in which reflux of gastric or intestinal contents occurs into the esophagus and results in patient complaints of heartburn and/or other symptoms (e.g., as a sensation of “burping up” stomach contents or as the taste of bitter contents in the mouth). The classic symptom of reflux is “heartburn,” which commonly is described as a pain in the center of the chest that, at times, might mimic cardiac angina. An estimated 44% of adults in the United States experience heartburn at least once per month and 7% of people have daily GERD symptoms. Acid reflux is also associated with asthma and when this occurs, is called “atypical GERD.” These symptoms, when accompanied by dysphagia (swallowing difficulty or a sensation of food sticking in the throat), odynophagia (severe pain on swallowing), or persistent nocturnal symptoms, usually are sufficient to make the patient aware of the need for medical attention. GERD symptoms are not necessarily associated with inflammation and tissue damage to the esophagus (esophagitis). An endoscopic examination is necessary to diagnose esophagitis. Esophagitis commonly is graded using the Savary-Miller system. This system grades esophagitis from grade 1 (mild) to grade 4 (severe, erosive changes). Although it is possible for esophagitis to be present in the absence of symptoms (and vice versa), it is important to understand that symptoms are poorly correlated with the grade of esophagitis.104–107 Complications associated with the reflux of gastric material into the esophagus include strictures, hemorrhage, perforation, aspiration, and the development of Barrett’s esophagus (see following discussion). Infants (up to 18 months of age) can experience a form of GERD that is characterized by postmeal regurgitation or small volume vomiting, which can occur several times per day due to a poorly functioning sphincter. Esophagitis is usually absent in these children, but a small percentage may develop chronic GERD. This form of GERD may respond very well to liquid formulations of gastric motility–stimulating agents. True motility dysfunction resulting in slowed or absent esophageal food transit times are uncommon in adults with GERD. UPPER GASTROINTESTINAL DISORDERS Pathogenesis The esophagus functions to transport food from the mouth to the stomach and to prevent the retrograde flow (i.e., “backflow”) of GI contents. As food enters the esophagus, the LES opens and remains open until the peristaltic contractions of esophageal muscles have swept the food into the stomach. Contraction of the lower esophageal sphincter after the passage of food into the stomach serves to prevent the reflux of gastric contents back into the esophagus. The development of GERD is associated with a disruption of the balance between defensive mechanisms (e.g., LES closure, esophageal peristalsis) and aggressive factors (e.g., acid, pepsin, bile salts).108 Three pathophysiologic mechanisms predispose a patient to reflux: (1) a spontaneous transient or sustained relaxation of the lower esophageal sphincter, (2) a low resting LES pressure, and (3) increased gastric pressure (e.g., bending over, pregnancy, obesity).109 All these pathophysiologic mechanisms can occur in normal healthy individuals, but additional variables may contribute to the development of esophageal damage. For example, the amount of acid and pepsin that refluxes within a 24-hour period, the length of time that acid and pepsin remain in the esophagus, and the natural sensitivity of the esophageal mucosa to damage by aggressive forces all determine the susceptibility of an individual to GERD.104 Clinical Presentation 19. R.L., a 37-year-old, 100-kg, 68-inch tall man, has been experiencing heartburn for approximately 4 to 6 weeks. The pain usually occurs after dinner while he is lying on the couch watching television and often is accompanied by regurgitation of a warm, foul-tasting fluid into his mouth. He also has experienced these symptoms, after his night-time snack of chocolate ice cream, soon after he goes to bed. He smokes 1 to 2 packs/day of cigarettes and drinks 2 beers every evening while watching television. His symptoms are relieved by Mylanta, which he drinks directly from the bottle. What symptoms does R.L. have that are consistent with GERD? Retrosternal burning, commonly referred to as heartburn, is the characteristic symptom of GERD and is caused by the contact of refluxed GI material with an inflamed esophageal mucosa.104 The epigastric pain can be angina-like and is described as a burning, substernal pain that travels up the esophagus toward the throat.104 In some patients, especially the elderly, no heartburn or chest pain is experienced and coughing or wheezing may be the only symptoms noted. However, the absence of symptoms does not necessarily imply the absence of esophageal damage. Symptoms of GERD often are episodic because GI contents are not refluxed continuously.104 The frequency of heartburn symptoms and the presence of esophagitis are correlated with the duration and intensity of acid exposure (often defined in terms of percentage of time per 24 hours that the pH is 4.0). Symptoms of GERD may be aggravated by certain foods, specific body positioning, or drugs. Foods with high-fat content (e.g., ice cream) can lead to GERD symptoms because these foods decrease the LES pressure. Other food products such as spices, onions, citric juices, and coffee can contribute to the development of GERD by direct mucosal irritation.109 • 27-19 Although symptoms of esophageal reflux can occur in any body position at any time, many patients experience symptoms primarily when they are supine or when bending over. The swallowing of saliva, water, or antacids (e.g., the Mylanta ingested by R.L.) frequently relieves the pain. R.L.’s symptoms of heartburn, the regurgitation of gastric contents soon after eating, and the association of these symptoms when he lies on the couch after dinner and when he goes to bed at night are all consistent with GERD. R.L.’s ingestion of two beers after dinner and of chocolate ice cream before bedtime and the apparent temporal relationship of these activities to the appearance of epigastric pain also are consistent with GERD. Concurrent drug therapy (e.g., alcohol, -adrenergic agonists, -adrenergic agonists, verapamil, diazepam, dopamine, meperidine, progesterone, prostaglandins, theophylline) also can exacerbate GERD.110 Treatment Goals 20. What are the therapeutic goals for the treatment of R.L.’s GERD? The therapeutic goals for GERD are: alleviate pain and symptoms, diminish the frequency and duration of esophageal reflux, promote healing of esophagitis (if present), avoid complications, prevent recurrence, and provide cost-effective therapy.110,111 One concern of uninvestigated GERD is Barrett’s esophagus (histologic change whereby the lower esophageal tissue begins to resemble the columnar epithelium similar to the lining of the stomach). Barrett’s esophagus predisposes the patient to the development of esophageal cancer: the more frequent, more severe, and longer-lasting the symptoms of reflux, the greater the risk. Persons with longstanding (>20 years’ duration) and severe symptoms of reflux (reflux-symptom score of 4.5) were 43.5 times more likely (95% confidence interval) for development of esophageal adenocarcinoma.110 Lifestyle Changes 21. What nondrug measures can improve R.L.’s symptoms? Initially, all patients should change their lifestyle to eliminate factors that can exacerbate reflux.110,112 Weight loss and loose-fitting clothes are recommended because obesity and tight clothes may exacerbate reflux by continuously increasing the gastroesophageal pressure gradient.112 Cigarette smoking directly provokes acid reflux and decreases lower esophageal sphincter pressure; efforts should be initiated to discontinue cigarette smoking.113 Foods that lower esophageal sphincter pressure (e.g., chocolate, alcohol, peppermint, fatty meals), irritate the gastric mucosa (e.g., spicy food, citric juices), or stimulate acid secretion (e.g., cola, beer) should be avoided. Most experts believe lifestyle modifications provide improved symptoms control in only approximately 20% of patients.104,110 These measures are widely promoted in the lay press and on web sites that discuss GERD. It is likely that many patients have already tried lifestyle modification before seeking medical help, thus many clinicians fail to find significant improvements in symptoms when they ask patients to use these measures. Despite these doubts about effectiveness, 27-20 • GASTROINTESTINAL DISORDERS all recommended lifestyle measures should be used by patients with GERD. Lifestyle modification as a sole treatment has never been demonstrated to heal esophagitis and should not be used as the sole treatment in these patients.104 R.L. should be instructed to lose weight, cease smoking, avoid lying on the couch in a position that increases gastric pressure, and avoid foods (chocolate ice cream, beer) that precipitate symptoms or decrease the LES pressure. He also should avoid meals 2 hours before lying down. R.L.’s symptoms appear to be the most troublesome while lying on the couch when he is watching television and at night. For patients like R.L., elevating the head of the bed with 6- to 8-inch blocks or sitting in an upright position might be helpful in enhancing the gravitational flow of gastric contents away from the esophagus.112,113 Drug Therapy Because patients with GERD have frequent reflux episodes of acid, effective inhibition of gastric acid secretion is necessary to facilitate healing and relieve symptoms.102,110,116 Antisecretory drugs, such as H2-receptor antagonists and PPIs, are generally used and the choice of drug is based on the severity of the disease.104,110 ANTACIDS Antacids can provide relief of mild symptoms associated with GERD. Antacids have short durations of action and do not promote healing of esophagitis.104,110 Consequently, antacids are used primarily as adjunctive agents to provide daytime symptomatic relief of GERD. H2-ANTAGONISTS H2-antagonists can be effective for management of mild GERD when used in full-sized doses. Healing rates with H2antagonists for patients with mild esophagitis are significantly better than placebo.111,117 The severity of the disease, the dose of the drug, and the duration of therapy affect the response to H2-antagonists. For example, endoscopic healing was observed in 65% of patients with moderate esophagitis (grade II), in contrast to only 15% of patients with severe esophagitis (grade III) after 8 weeks of ranitidine therapy.96,110 Gastric acid suppression throughout the day is important in the treatment of reflux disease. As a result, H2-antagonists administered in daily divided doses provide superior symptom relief and healing than does once-daily dosing.110 Duration of H2-antagonist treatment is another major determinant of esophagitis healing rates.116 In one multicenter trial, 57% of patients with esophagitis were healed after 6 weeks of therapy, and 70% healed after 12 weeks of therapy. In another study, 45% of patients taking nizatidine experienced esophagitis healing after 6 weeks of therapy and 80% were healed after 12 weeks.118 In both studies, symptom relief occurred earlier than esophagitis healing, confirming the poor correlation between symptom relief and presence of esophagitis. In addition, it is clear that esophagitis in some patients does not heal in response to H2-antagonists. Some investigators attribute this lack of response to H2-antagonist drug tolerance.110 PROTON PUMP INHIBITORS PPIs such as omeprazole (Prilosec), lansoprazole (Prevacid), rabeprazole (Aciphex), pantoprazole (Protonix), or es- omeprazole (Nexium), which can inhibit gastric acid secretion for a sustained period, are highly effective in the treatment of GERD. Normal, once-daily doses of PPIs for 4 weeks will heal approximately 85% to 90% of patients with the mild esophagitis and approximately 60% to 80% of patients with severe esophagitis (grade IV).104,110,111 Higher doses (usually twice the “normal” dose sometimes given in divided doses twice daily) are sometimes required to heal esophagitis in patients with more severe erosive disease. In clinical trials, PPIs heal esophagitis quicker and more effectively than H2-antagonists. In one randomized, blinded, controlled study, healing occurred in 67% of patients after 4 weeks of omeprazole (20 mg daily) compared with 31% of patients with ranitidine (150 mg twice a day).119 After 8 weeks, the healing rates increased further to 85% and 50% with omeprazole and ranitidine therapy, respectively. The higher healing rate for omeprazole also was accompanied by a more rapid improvement of symptoms. Omeprazole was notably more effective than ranitidine in patients with severe esophagitis.119 PPIs are also effective in healing esophagitis refractory to H2-antagonists. After 12 weeks of therapy, 80% of patients with severe esophagitis experienced healing with 20 to 40 mg/day of omeprazole despite previous failures to prolonged therapy with H2-receptor antagonists.119,120 In another multicenter, randomized, blinded study, lansoprazole (30 mg/day) was more effective in healing erosive esophagitis in patients who were resistant to standard doses of H2-receptor antagonists than continuation of ranitidine (150 mg twice a day).121 The healing rate for lansoprazole was 89% at 8 weeks compared with 38% for ranitidine (P 0.001). In comparative trials, PPIs have been shown to heal all grades of esophagitis in at least 65% to 90% of patients in 4 to 8 weeks. When one compares esophagitis healing, PPIs are generally equally effective in healing mild to moderate reflux esophagitis. In one multicenter trial that evaluated healing of erosive esophagitis, lansoprazole 30mg daily and omeprazole 20 mg had similar healing rates at 8 weeks. Patients taking lansoprazole (30 mg/day) had significantly better pain relief (by 11%) than the omeprazole group in the first week.122 Similar results have been seen with rabeprazole and esomeprazole (40mg/day) when compared with omeprazole (20 mg/day). SUCRALFATE Sucralfate (Carafate) appears to be effective in resolving mild cases of esophagitis but is less effective in management of severe disease.117,123 As a result, sucralfate is not as widely used as the acid suppressants to manage GERD. METOCLOPRAMIDE Metoclopramide (Reglan) stimulates the motility of the upper GI tract without affecting gastric acid secretion. When administered as 10 mg, four times a day, 30 to 60 minutes before meals and at bedtime, it increases gastric peristalsis, which leads to accelerated gastric emptying.5 Duodenal peristalsis is increased and intestinal transit time is decreased in patients with GERD.124 Metoclopramide also increases the LES resting tone within 1 to 3 minutes following IV administration and in 30 to 60 minutes after oral administration.116 Metoclopramide’s adverse effect profile (e.g., drowsiness, diarrhea, abdominal cramps, extrapyramidal reactions) and the UPPER GASTROINTESTINAL DISORDERS general lack of GI motility dysfunction in adult patients with GERD significantly limit the usefulness of this medication for the treatment of GERD. Cisapride (Propulsid) is no longer commercially available. The manufacturer (Janssen) has a special investigational limited access program for cisapride distribution which is primarily intended for patients with diabetic gastroparesis. Tegaserod, a 5-HT4 receptor agonist, improves LES tone and will reduce the esophageal exposure to gastric acid when compared to placebo, but its effective use in GERD has not yet been demonstrated.125 22. R.L. has lost approximately 5 kg of body weight over the past 6 weeks and has discontinued drinking two beers each night and eating all spicy foods. However, he continues to smoke cigarettes despite efforts at cessation. His symptoms of reflux have continued despite his regular use of nonprescription cimetidine tablets, which were purchased at his local pharmacy. What drug therapy is appropriate for the treatment of his GERD at this time? The traditional treatment of GERD has been based on the premise of incremental increases in the intensity of treatment options (i.e., begin first with lifestyle management and nonprescription doses of an H2-antagonist, which can be replaced, if needed, by nonprescription omeprazole or prescription strength H2-antagonists, and ultimately by increasing doses of a PPI in lieu of the H2-antagonist). Even though R.L. has modified his lifestyle, with the exception of cigarette cessation, his condition has not responded to nonprescription cimetidine. In addition he lost weight. He should be referred for endoscopy to determine if he has an esophageal stricture or esophageal adenocarcinoma which can obstruct his esophagus resulting in dysphagia and weight loss. If esophagitis is seen on endoscopy, he will likely be placed on a PPI. This is because PPIs are more effective than standard and high (double)-dose H2-antagonists in symptom relief, healing, and maintenance of remission of esophagitis. The superiority of PPIs presumably are related to the inability of even high-dose H2-antagonists to control acid secretion and possibly also due to the development of tolerance to H2antagonists.The PPIs provide quicker relief of symptoms than H2-antagonists and, as a result, are possibly more of an incentive for patients to adhere to therapy.126 Maintenance Therapy 23. Esophagitis was noted in R.L.’s endoscopy, and he was prescribed omeprazole 20 mg twice daily. Eight weeks later, a repeat endoscopy revealed the esophagitis had healed, and the omeprazole was stopped. Since that time, he has begun to reexperience some mild symptoms occasionally. Is maintenance therapy recommended for R.L.’s occasional symptoms? Unfortunately, even after complete healing, a large percentage of patients experience a recurrence of symptoms within 6 to 12 months after therapy is discontinued.5,110,116,122,123,127 Therefore, maintenance therapy is often required. One recent study demonstrated that continuous maintenance treatment with a daily PPI is the most effective treatment strategy. This study compared the recurrence rates of reflux esophagitis over a 12-month period with either omeprazole 20 mg/day, omeprazole 20 mg three times a week, or ranitidine 150 mg twice daily. At 12 months, endoscopy showed sustained remission of disease in 89% of patients receiving omeprazole • 27-21 daily, in 32% receiving omeprazole three times a week, and in 25% receiving ranitidine.128 Trials using other PPIs have found similar results. Maintenance therapy with a PPI should be initiated in R.L. after further medical workup because the risk of morbidity can be high and GERD often is a chronic condition.126 STRESS-RELATED MUCOSAL BLEEDING Acute stress-related mucosal lesions (SRML) are distinctly different from chronic PUD.129 They occur in critically ill patients, are usually unrelated to a previous history of ulcer disease, and are seen within 24 to 48 hours of admission to hospital.130 Multiple superficial ulcerative lesions can develop in the proximal stomach along the boundaries of the acid-secreting mucosa and the duodenum.129,130 Approximately 50% of these lesions bleed, but because they are superficial, significant bleeding will occur in only 5% to 20% of untreated patients in intensive care units.114 Patients in whom GI bleeding develops have a higher mortality rate than those critically ill patients who do not have GI bleeding.130 During the early 1970s, stress ulcers and their complications had reached near epidemic proportions.114 However, the occurrence of stress ulcer bleeding has decreased to 2% of the intensive care patient population due to widespread use of agents that prophylactically neutralize gastric acids and advanced medical care.131 Pathogenesis Stress ulcers primarily occur because impaired protective mechanisms cause an imbalance between aggressive and defensive forces resulting in mucosal damage. Although hydrogen ions are necessary for the development of stress ulcers, large quantities of acid secretion are not necessary.114 In fact, critically ill patients usually secrete normal amounts of gastric acid.130 For example, trauma patients secrete normal or decreased amounts, whereas hypersecretion is found in patients with sepsis, CNS injuries, or small bowel resections.131,132 Despite the critical role of gastric acid and other aggressive factors in the pathogenesis of stress ulceration, mucosal defenses also are important and these are often compromised in the critically ill patient. Gastric mucosal blood flow is diminished in patients with hemorrhagic, cardiogenic, and septic shock,114 resulting in a number of unfavorable events: (1) decreased nutrient and oxygen delivery, (2) inability of the mucosa to remove or neutralize gastric acids, and (3) a decrease in bicarbonate efflux into the gastric epithelium.130 During stressful situations, there also is a decreased rate of proliferation and cellular turnover of the gastric mucosa.114 Furthermore, in many critically ill patients, diffusion of bile salts backward from the duodenum into the stomach and high concentrations of urea in the blood (e.g., renal failure) can disrupt the gastric barrier and contribute to stress injury. The lack of prostaglandin and mucous formation in the critically ill also may contribute to the development of stress-related damage. Risk Factors 24. M.L., a 64-year-old woman who was admitted with diverticular disease, is transferred to the intensive care unit (ICU) because of her deteriorating respiratory condition and septic 27-22 • GASTROINTESTINAL DISORDERS shock. She has no significant past medical history and was a healthy individual. She is in respiratory distress with a respiratory rate of 36 breaths/min, temperature of 104°F, heart rate of 120 beats/min, and a blood pressure (BP) of 60/40 mm Hg. Relevant laboratory tests include the following: sodium (Na), 147 mEq/L; potassium (K), 5.6 mEq/L; chloride (Cl), 119 mEq/L; bicarbonate, 12 mEq/L; blood urea nitrogen (BUN), 57 mg/dL; serum creatinine (SrCr), 2.7 mg/dL; and white blood cell (WBC) count, 36,000 cells/mm3. Her respiratory status deteriorates to a point that endotracheal intubation is necessary. In addition to empiric antibiotics and fluids, treatment for stress ulcers is being contemplated. M.L has no history of PUD. What are the risk factors for stress ulcers that are present in M.L? [SI units: Na, 147 mmol/L; K, 5.6 mmol/L; Cl, 119 mmol/L; bicarbonate, 12 mmol/L; BUN, 20.3 mmol/L; WBC count, 36,000 106 cells/L] Numerous risk factors have been identified for stress ulcers and these include shock, burns (>30% of the body), multiple organ failure, trauma (intra-abdominal or thoracoabdominal injuries), sepsis (intraperitoneal or pulmonary infections), coagulopathy, and CNS injury. However, in a prospective multicenter study that evaluated potential risk factors for stress ulceration in 2,252 critically ill medical ICU patients, the only independent risk factors for GI bleeding were respiratory failure and coagulopathy. They concluded that prophylaxis against stress ulcers may be required only in critically ill patients who have a coagulopathy or require mechanical ventilation for 48 hours.114 In another study, respiratory failure (mechanical ventilatory support 24 hours) and high-dose corticosteroid administration (250 mg/day of hydrocortisone) was associated with an increased risk of stress ulcer bleeding.131 M.L. currently is in septic shock and will require mechanical ventilation; therefore, she should receive stress ulcer prophylaxis. Treatment 25. What can be done to prevent the complications associated with SRML in patients like M.L.? Despite the low occurrence of serious bleeding in critically ill patients, when it occurs, the accompanying mortality is very high. Aggressive prophylactic therapy may prevent significant complications and reduce both morbidity and mortality rates. Because alteration in defense mechanisms predisposes a patient to stress ulceration and bleeding, the most important approach is to treat the underlying disease state and physiologic conditions. The keystones are early fluid resuscitation, immediate oxygenation, early hemodynamic stabilization, prevention of infections, and effective analgesia and sedation.114 For example, improving blood flow by correcting shock should be a priority in the management of M.L.114,131 Other important measures are maintaining adequate nutrition and respiratory support. Some studies suggest that enteral nutrition may aid in the prevention of GI bleeding.132 Acid-base imbalances and uremia should be corrected because of their propensity for injury to the gastric mucosa. M.L. has evidence of both a metabolic acidosis (HCO3, 12 mEq/L) and renal insufficiency (SrCr, 2.7 mg/dL). Adequate neutralization of gastric acid is of paramount importance because stress-associated ulceration does not occur in the absence of acid, and if the intragastric pH is maintained between 3.5 and 4, the frequency of bleeding is lowered in stressed patients. At a pH of 4.5, pepsin is inactivated, whereas 99.9% of acid is neutralized at a pH of 5. In vitro observations demonstrate that when the gastric pH is 5 to 7, coagulation function and platelet aggregation are impaired.130 Therefore, therapy should be directed at maintaining the gastric pH between 3.5 and 4 to prevent SRML. 26. What pharmacologic therapy can be used to prevent SRML in M.L.? Aggressive antacid therapy (e.g., Mylanta 30 to 60 mL every hour) very effectively maintains the gastric pH above 3.5.131 Numerous studies have compared H2-antagonists with antacids for prevention of stress-related bleeding. Most have found pH control to be superior with antacids (when antacids are given hourly), but the rate of significant bleeding was not found to be different between these two treatment regimens.133 Although antacids are effective in preventing stress ulcers, they are inconvenient to administer and are associated with accumulation of magnesium, aluminum, or calcium. Diarrhea can also occur. H2-Antagonists H2-antagonists effectively prevent stress ulcers. In an analysis of data from 16 prospective, randomized trials, cimetidine was as effective as antacids in preventing significant stress ulcer bleeding.133 Another prospective, randomized trial compared the efficacy of several H2-receptor antagonists (cimetidine, famotidine, and ranitidine) and antacids in the prevention of stress ulceration.133 In a double blinded trial comparing cimetidine constant infusion (50 to 100 mg/hr) with placebo designed to prevent GI bleeding from SRML in critically ill patients, the cimetidine group had significantly fewer bleeding episodes.134 Proton Pump Inhibitors (PPIs) PPIs are potent acid suppressant drugs, and in contrast to H2receptor antagonists, PPIs inhibit both histamine and vagally induced gastric acid secretion. Because of this, these agents should be expected to be useful in the management of stress ulcer patients. Data regarding use of PPIs to prevent bleeding associated with SRML is summarized below. 27. M.L. is to be started on ranitidine. How should it be administered? Because the goal is to maintain the gastric pH 4.0 for the prevention of SRML and 7.0 for the treatment of bleeding ulcers, the proper administration of these agents is critical (see Table 27-6 for recommended dosing). Intermittent bolus injection has been used; however, this method of administration is labor intensive, and a continuous IV infusion of the H2receptor antagonists is more effective in maintaining the gastric pH above 4.135–137 Dosages should be adjusted according to the severity of the patient’s illness, renal function, and the intragastric pH. The intragastric pH can be determined with an indwelling probe or by measuring the pH of an NG aspirate. Signs of bleeding (e.g., significant bleeding out the nasogastric tube, low blood pressure, dropping Hct and Hgb) also should be assessed constantly. UPPER GASTROINTESTINAL DISORDERS Table 27-6 • 27-23 Stress-Related Mucosal Bleeding Prevention: Regimens and Doses Agent Dose and Frequency of Administration Antacid 30 mL PO/NG Q 1–2 hr No Cimetidine 300 mg IV Q 6–8 hr 300 mg IV loading dose then 50 mg/hr continuous IV infusion No Yes Famotidine 20 mg IV Q 12 hr 1.7 mg/hr continuous IV infusion No No Ranitidine 50 mg IV Q 6–8 hr 6.25 mg/hr continuos IV infusion No No Sucralfate 1 g PO/NG Q 4–6 hr No Omeprazole FDA Approved b No b 20–40 mg PO/NG Q 24 hr Lansoprazole 30–60 mg PO/NG Q 24 hr No Pantoprazole 40 mg IV Q 12–24 hr No a For prevention of SRMB. By suspension in bicarbonate. IV, intravenous; NG, by nasogastric tube; PO, by mouth. b Sucralfate 28. Is there any role for sucralfate in treating M.L.? In the critically ill patient receiving stress ulcer prophylaxis with acid-suppressing medications or neutralizing agents, growth of Gram-negative bacteria is common.115 Because an elevated gastric pH promotes proliferation of bacteria in the stomach, aspiration of these organisms may be important in the pathogenesis of nosocomial pneumonia. Sucralfate does not alter gastric pH significantly but has generally been found to be effective in preventing SRML associated bleeding.138,139 Several investigators have evaluated the association between SRML prophylaxis nosocomial pneumonia in the critically ill patient.115,138,139 Most studies have failed to demonstrate a significant difference in development of nosocomial pneumonia between sucralfate and H2-antagonists.115,138,139 A more recent study found that in critically ill patients requiring mechanical ventilation there was no significant difference in the occurrence of pneumonia, and ranitidine was more effective than sucralfate in reducing the rate of GI bleeding.140 Sucralfate is available as a suspension and administration through M.L.’s NG tube would be simple; however, M.L. has decreased renal function and sucralfate can cause aluminum toxicity in renally impaired patients. Therefore, sucralfate may not be a good therapeutic option for M.L. A continuous IV infusion of an H2-antagonist may be appropriate in this situation. The dose of the H2-receptor should be adjusted ac- cording to M.L.’s calculated creatinine clearance (i.e., ClCr 10 mL/ min, use 0.8 mg/hr famotidine). 29. Is there a role for PPIs in the prevention of SRML for M.L.? PPIs administered by NG tube have been shown to provide sufficient acid suppression to prevent SRML.98–102 In one study comparing an omeprazole suspension (in bicarbonate) administered in the NG tube with ranitidine, the NG omeprazole group had fewer GI significant bleeding episodes.135 These authors had a very broad definition of GI bleeding (Hgb drop of 2), and the GI bleeding episodes noted may have been from non-GI sources; thus, many believe the difference noted may not be reproducible in actual practice. It is likely that NG PPIs are effective, but superiority over other regimens needs to be confirmed in further studies. Intravenous PPIs have been used in numerous studies, but blinded, randomized trials in SRML prophylaxis have not yet been published. Thus, comparative efficacy with other options is not yet possible. Intravenous omeprazole and pantoprazole are available in Canada and most of Europe, but only pantoprazole is available for intravenous use in the US. Several trials are currently ongoing comparing IV PPIs with H2antagonists, but results are not available yet. It is likely they are effective at preventing SRML associated GI bleeding, but further work is necessary to determine comparative efficacy of any PPIs with the other available options. REFERENCES 1. Corazziari E. New Rome criteria for functional gastrointestinal disorders. Dig Liver Dis 2000; 32(Suppl 3):S233. 2. Hsu P et al. Eradication of Helicobacter pylori prevents ulcer development in patients with ulcer-like functional dyspepsia. Aliment Pharmacol Ther 2001;15:195. 3. Johnsen R et al. Prevalence of endoscopic and histologic findings in subjects with and without depression. Br Med J 1991;302:749. 4. Rosenstock S et al. Risk factors for peptic ulcer disease: a population based cohort study comparing 2416 Danish adults. Gut 2003;52:186. 5. Dent J on Behalf of the Genval Workshop group. An evidenced based appraisal of reflux diagnosis and management. The Genval Workshop Report. Gut 1999;44(Suppl 2):S1. 6. Lagergren J et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825. 7. Barbara L et al. Definition and investigation of dyspepsia: consensus of an international ad hoc working party. Dig Dis Sci 1989;34:1272. 27-24 • GASTROINTESTINAL DISORDERS 8. Delaney BC et al. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2003: DC001961. 9. Duggan AE et al. Does initial management of patients with dyspepsia alter symptom response and satisfaction? Gastroenterology 1999;(S4): G0654. 10. Heaney A et al. A prospective randomised trial of a “test and treat” policy versus endoscopy-based management in young H. pylori positive patients with ulcer-like dyspepsia. Gut 1999;45:186. 11. Lassen AT et al. H. pylori “test and treat” or prompt endoscopy for dyspeptic patients in primary care. A randomized controlled trial of two management strategies. Gastroenterology 1998;114:G0853. 12. Mason I et al. The management of acid-related dyspepsia in general practice: a comparison of omeprazole versus an antacid alginate/ranitidine management strategy. Aliment Pharmacol Ther 1998;12:263. 13. Meinche-Schmidt V, Krag E. Antisecretory therapy in 1017 patients with ulcer-like dyspepsia in general practice. Eur J Gen Pract 1997;3:125. 14. Jones RH, Baxter G. Lansoprazole 30 mg daily versus ranitidine 150 mg b.d. in the treatment of acidrelated dyspepsia in general practice. Aliment Pharmacol Ther 1997;11:541. 15. McColl K et al. Symptomatic benefit from eradicating H. pylori infection in patients with nonulcer dyspepsia. N Engl J Med 1998;339:1869. 16. Blum AL et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med 1998;339:1875. 17. Watanabe Y et al. Epidemiological study of PUD among Japanese and Koreans in Japan. J Clin Gastroenterol 1992;15:68. 18. Sonnenberg A. Temporal trends and geographical variation of peptic ulcer. Aliment Pharmacol Ther 1995;9(Suppl 2):3. 19. Katz J. The course of peptic ulcer disease. Med Clin North Am 1991;75(4):831. 20. Rosenstock S et al. Risk factors for peptic ulcer disease: a population based prospective cohort study comprising 2416 Danish adults. Gut 2003;52:186. 21. Katz J. Acid secretion and suppression. Med Clin North Am 1991;75(4):877. 22. Wallace JL. Mucosal defense—new avenues for treatment of ulcer disease? Gastroenterol Clin North Am 1990;19(1):87. 23. Schiessel R et al. Mechanisms of stress ulceration and implications for treatment. Gastroenterol Clin North Am 1990;19(1):101. 24. Shiotani A, Graham DY. Pathogenesis of gastric and duodenal ulcer. Med Clin North Am 2002;86:1447. 25. Graham DY. Treatment of peptic ulcers caused by Helicobacter pylori. N Engl J Med 1993;328:349. 26. Marshall B. Unidentified curved bacillum in gastric epithelium in active chronic gastritis. Lancet 1983;1:1237. 27. Marwick C. Helicobacter: new name, new hypothesis involving type of gastric cancer. JAMA 1990; 254:2724. 28. Drumm B et al. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med 1990; 322:359. 29. Ateshkadi A et al. Helicobacter pylori and peptic ulcer disease. Clin Pharm 1993;12:34. 30. Valle J et al. Disappearance of gastritis after eradication of Helicobacter pylori: a morphometric study. Scand J Gastroenterol 1991;26:1057. 31. Dunn BE. Pathogenic mechanisms of Helicobacter pylori. Gastroenterol Clin North Am 1993; 22(1):43. 32. Mc Gowan CC et al. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterol 1996;110:926. 33. Kuipers EJ et al. The prevalence of H. pylori in peptic ulcer disease. Aliment Pharmacol Ther 1995;9(Suppl 2):59. 34. Reinbach DH et al. Acute perforated duodenal ulcer is not associated with Helicobacter pylori infection. Gut 1993;34:1344. 35. Graham DY. NSAIDs, Helicobacter pylori, and Pandora’s Box. N Engl J Med 2002;347:2162. 36. National Institutes of Health (NIH) Consensus Development Panel on Helicobacter pylori in peptic ulcer disease. JAMA 1994;272:65. 37. Feldman M, Burton ME. Histamine-receptor antagonists standard therapy for acid-peptic disease. N Engl J Med 1990;323(24):1672. 38. Hurwitz A, Carter CA. The pharmacology of antiulcer drugs. Ann Pharmacother 1989;23:S10. 39. Lin JH. Pharmacokinetic and pharmacodynamic properties of histamine H2-receptor antagonist. Clin Pharmacokinet 1991;20(3):218. 40. Sax MJ. Clinically important adverse effects and drug interactions with H2-receptor antagonists: an update. Pharmacotherapy 1987;7(6 Pt. 2):110S. 41. Humphries TJ, Merritt GJ. Drug interactions with agents used to treat acid related disorders [Review]. Aliment Pharmacol Ther 1999;13(Suppl 3):18. 42. Nazario M. The hepatic and renal mechanisms of drug interactions with cimetidine. Drug Intell Clin Pharm 1986;20:342. 43. Kosoglou T, Vlasses PH. Drug interactions involving renal transport mechanisms: an overview. Ann Pharmacother 1989;23:116. 44. Christian C et al. Cimetidine inhibits renal procainamide clearance. Clin Pharm Ther 1984; 36:221. 45. Caballeria J et al. Effects of cimetidine on gastric alcohol dehydrogenase activity and blood ethanol levels. Gastroenterology 1989;96:388. 46. Raufman JP et al. Histamine2-receptor antagonists do not alter serum ethanol levels in fed, nonalcoholic men. Ann Intern Med 1993;118:488. 47. Howden CW, Hunt RH. The relationship between suppression of acidity and gastric ulcer healing rates. Aliment Pharmacol Ther 1990;4:25. 48. Jones R, Bytzer P. Acid suppression in the management of gastro oesophageal reflux disease: an appraisal of treatment options in primary care [Review]. Aliment Pharmacol Ther 2001;15:765. 49. Massoomi F et al. Omeprazole: a comprehensive review. Pharmacother 1993;13(1):46. 50. Langtry HD, Wilde MI. Lansoprazole: an update of its pharmacological properties and clinical efficacy in the management of acid-related disorders. Drugs 1997;3:473. 51. Lanza F et al. Double-blind comparison of lansoprazole, ranitidine and placebo in the treatment of acute duodenal ulcer. Am J Gastroenterol 1994;89:1191. 52. Ramakrishnan A, Katz PO. Overview of therapy for gastroesophageal reflux disease. Gastrointest Clin North Am 2003;13:57. 53. Richardson P et al. Proton pump inhibitors: pharmacology and rationale for use in gastrointestinal disorders. Drugs 1998;56(3):307. 54. Pactoflickovic D et al. Acid inhibition in the first day of dosing: a comparison of four PPIs. Aliment Pharmacol Ther 2003;17:1507. 55. Hatelbakk JG. Review article: gastric acidity— comparison of esomeprazole with other proton pump inhibitors. Aliment Pharmacol Ther 2003;17(Suppl1):10. 56. Maton PN. Omeprazole. N Engl J Med 1991; 324:965. 57. Naesdal J et al. Pharmacokinetics of 14C omeprazole in patients with impaired renal function. Clin Pharmacol Ther 1986;40:344. 58. Geeson LB, Triadafilopoulos F. Proton pump inhibitors and therapeutic drug interactions: an evidenced based approach. Eur J Gastro Hepatol 2001;13:611. 59. Johnson TJ, Hedge DD. Esomeprazole, a clinical review. Am J Health Syst Pharm 2002;59:1333. 60. Shorrock CJ, Rees WDW. Effect of sucralfate on human gastric bicarbonate secretion and local prostaglandin E2 metabolism. Am J Med 1989;86(Suppl 6A):2. 61. Martin F. Sucralfate suspension 1 g four times per day in the short-term treatment of active duodenal ulcer. Am J Med 1989;86(Suppl 6A):104. 62. Van Ness MM. Antacids. In: Van Ness MM, ed. Handbook of Gastrointestinal Drug Therapy. Boston: Little, Brown Co., 1989:7. 63. Schubert T. Twice-daily sucralfate dosing to heal acute duodenal ulcer. Am J Med 1989;86(Suppl 6A):108. 64. Vecu A et al. Amoxicillin, clarithromycin, and either sucralfate or pantoprazole for eradication of H pylori in duodenal ulcer. Wein Clin Wachlen 2001;113:935. 65. Walt RP, Langman MJS. Antacids and ulcer healing—a review of the evidence. Drugs 1991; 42(2):205. 66. Nix DE et al. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin Pharmacol Ther 1989;46:700. 67. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection: Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998;93:2330. 68. Barthel JS, Everett ED. Diagnosis of Campylobacter pylori infections: the “gold standard” and the alternatives. Rev Infect Dis 1990;12(Suppl 1):S107. 69. Rostom A et al. Prevention of NSAID induced gastroduodenal ulceration (Cochrane Review). Cochrane Database Syst Rev 2002;(4):C0002296. 70. Labenz J et al. Amoxicillin plus omeprazole versus triple therapy for eradication of Helicobacter pylori in duodenal ulcer disease: a prospective, randomized, and controlled study. Gut 1993;34:1167. 71. Taylor JL. Pharmacoeconomic comparison of treatments for the eradication of Helicobacter pylori. Arch Intern Med 1997;157:87. 72. Graham DY et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer: a randomized, controlled study. Ann Intern Med 1992;116:705. 73. Hentschel E et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med 1993;328:308. 74. Marshall BJ. Treatment strategies for Helicobacter pylori infection. Gastroenterol Clin North Am 1993;22:183 75. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. Helicobacter pylori in peptic ulcer disease. JAMA 1994;272:65. 76. Okam A et al. Relationship between anti-inflammatory drug use and Helicobacter pylori infection in bleeding or uncomplicated ulcers. J Gastroenterol Hepatol 2003;18:18. 77. Maton PN et al. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology 1989;97:827. 78. Jensen RT, Fraker DL. Zollinger-Ellison syndrome. JAMA 1994;271:1429. 79. Jensen RT. Basis for failure of cimetidine in patients with Zollinger-Ellison syndrome. Dig Dis Sci 1998;29:363. 80. Mellem H et al. Symptoms in patients with peptic ulcer and hematemesis and/ or melena related to the use of non-steroid anti-inflammatory drugs. Scand J Gastroenterol 1985;20:1246. 81. Graham DY et al. Prevention of NSAID-induced gastric ulcer with misoprostol multicentre, doubleblind, placebo-controlled trial. Lancet 1988;2: 1277. 82. McCarthy DM. Nonsteroidal anti-inflammatory drug-induced ulcers: management by traditional therapies. Gastroenterology 1989;196:662. 83. Kolts B, Achem S. Gastrointestinal side effects of nonsteroidal anti-inflammatory drug use. Hosp Formul 1992;27:36. 84. Neville D et al. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory agents. N Engl J Med 1998; 338:719. 85. Ehsanullah RSB et al. Prevention of gastroduodenal damage induced by non-steroidal anti-inflammatory drugs: controlled trial of ranitidine. Br Med J 1988;297:1017. 86. Robinson MG et al. Effect of ranitidine gastroduodenal mucosal damage induced by nonsteroidal anti-inflammatory drugs. Dig Dis Sci 1989;14:424. UPPER GASTROINTESTINAL DISORDERS 87. Taha AS et al. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal anti-inflammatory drugs. N Engl J Med 1996;334: 1435. 88. Agrawal M et al. Misoprostol compared with sucralfate in the prevention of nonsteroidal anti-inflammatory drug-induced gastric ulcer. Ann Intern Med 1991;115:195. 89. Graham DY et al. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Ann Intern Med 1993;119:257. 90. Raskin JB et al. Efficacy and safety of misoprostol in the prevention of NSAID-induced gastric ulcers—preliminary findings. Gastroenterology 1993;104:A177. 91. Hawkey CJ et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1998;338:727. 92. Hirschowitz BI. Zollinger-Ellison syndrome: pathogenesis, diagnosis, and management. Am J Gastroenterol 1997;92(Suppl 4):44S. 93. Jensen RT. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med 1983;98:59. 94. Wolfe MM, Jensen RT. Zollinger-Ellison syndrome. N Engl J Med 1987;317:1200. 95. Roy PK et al. Gastric Secretion in Zollinger-Ellison Syndrome: correlation with clinical expression, tumor extent and role in diagnosis. A prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine 2001;80:189. 96. Johnson NJ et al. Acute treatment of reflux oesophagitis: a multicenter trial to compare 150 mg ranitidine BD with 300 mg ranitidine QDS. Ailment Pharmacol Ther 1989;3:259. 97. Bonfils S et al. Prolonged treatment of ZollingerEllison syndrome by long-acting somatostatin. Lancet 1986;1:554. 98. Larson C et al. Bioavailability and efficacy of omeprazole given orally and by nasogastric tube. Digest Dis Sci 1996;41:475. 99. Sostek MB et al. Esomeprazole: nasogastric tube administration of the contents of an opened capsule suspended in water compared with oral administration in healthy volunteers [Abstract]. Pharmacotherapy 2002;22:1339. 100. Quercia RA et al. Stability of omeprazole in an extemporaneously prepared oral liquid. Am J Health-Syst Pharm 1997;54:1833. 101. McAndrews KL, Eastham JH. Omeprazole and lansoprazole suspensions for nasogastric administration. Am J Health-Syst Pharm 1999;56:81. 102. Ley LM et al. Bioavailability of crushed pantoprazole tablet after buffering with sodium hydrogen carbonate or magaldrate relative to the intact enteric coated pantoprazole tablet. Meth Find Exp Clin Pharmacol 2001;23:41. 103. Saeed ZA et al. Parenteral antisecretory drug therapy in patients with Zollinger-Ellison syndrome. Gastroenterology 1989;96:1393. 104. DeVault K, Castell D, for the Practice Parameters Committee of the American College of Gastroen- 105. 106. 107. 108. 109. 110. 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. 121. 122. 123. terology. Guidelines for the diagnosis and treatment of GERD. Am J Gastroenterol 1999;94:1434. Fennerty M et al. The diagnosis and treatment of gastroesophageal reflux disease in a managed care environment. Arch Intern Med 1996;156:477. Lambert R. Current practice and future perspectives in the management of gastro-oesphageal reflux disease [Review]. Aliment Pharmacol Ther 1997;11:651. Faubion W, Zein N. Gastroesophageal reflux in infants and children. Mayo Clin Proc 1998;73:166. Richter JE, Casterll DO. Gastroesophageal reflux. Ann Intern Med 1982;97:93. Gelfand MD. Gastroesophageal reflux disease. Med Clin North Am 1991;75:923. Deut J, on Behalf of the Genval Workshop Group. An evidenced based appraisal of reflux disease management: the Genval Workshop Report. Gut 1999;44(Suppl 2):S1. Kozarek RA. Complications of reflux esophagitis and their medical management. Gastroenterol Clin North Am 1990;19:713. Meining A, Classen M. The role of diet and lifestyle measures in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol 2000;95:2692. Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut 1990;31:4. Cook DJ et al. Risk factors for gastrointestinals bleeding in critically-ill patients. N Engl J Med 1994;330:377. Driks MR et al. Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. N Engl J Med 1987;317:1367. Sontag SJ. The medical management of reflux esophagitis. Gastroenterol Clin North Am 1990;19:683. Hatlebakk IG, Berstad A. Pharmacokinetic optimisation in the treatment of gastro-oesophageal reflux disease. Clin Pharmacokinet 1996;31:386. Quik RF et al. A comparison of two doses of nizatidine versus placebo in the treatment of reflux oesophagitis. Aliment Pharmacol Ther 1990;4:201. Klinkenberg-Knol EC et al. Double-blind multicentre comparison of omeprazole and ranitidine in the treatment of reflux oesophagitis. Lancet 1987;1:349. Sontag S et al. Omeprazole for esophagitis and ulcer refractory to H2-blockers. Gastroenterology 1989;94:A436. Feldman M et al. Treatment of reflux esophagitis resistant to H2-receptor antagonists with lansoprazole, a new H/K-ATPase inhibitor: a controlled, double-blind study. Am J Gastroenterol 1993:88;1212. Klok RM et al. Meta-analysis: comparing the efficacy of proton pump inhibitors in short term use. Aliment Pharmacol Ther 2003;17:1237. Ros J et al. Healing of erosive esophagitis with sucralfate and cimetidine: influence of pretreatment 124. 125. 126. 127. 128. 129. 130. 131. 132. 133. 134. 135. 136. 137. 138. 139. 140. • 27-25 lower esophageal sphincter pressure and serum pepsinogen I levels. Am J Med 1991;91(Suppl 2A):107S. McEvoy GK. Miscellaneous GI drugs. In: American Society of Hospital Pharmacists. AHFS Drug Information 2001. Bethesda, MD: American Society of Hospital Pharmacists, 2001. Kahrilis PJ et al. Effects of tegaserod on esophageal acid exposure in gastro-oesophageal reflux disease. Aliment Pharmcol Ther 2000;14:1503. Howden CW et al. Management of heartburn in a large randomized community-based study: comparison of four therapeutic strategies. Am J Gastroenterol. 2001;96:1679. Blum R. Lansoprazole and omeprazole in the treatment of peptic disorders. Am J Health-Syst Pharm 1996;53:1401. Howden CW et al. The rationale for continuous maintenance treatment of reflux esophagitis. Arch Intern Med 1995;155:1465. Levine BA. Pathophysiology and mechanisms of stress ulcer injury. Pharmacotherapy 1987;7(6 Pt. 2):90S. Tryba M, Cook D. Current guidelines on stress ulcer prophylaxis. Drugs 1997;54(4):581. Tamir BM et al. Prophylaxis for stress-related gastric hemorrhage in the medical intensive care unit. Ann Intern Med 1994;121:568. Ephgrave KS et al. Enteral nutrients prevent stress ulceration and increase intragastric volume. Crit Care Med 1990;18:621. Lamothe HP et al. Comparative efficacy of cimetidine, famotidine, ranitidine, and Mylanta in postoperative stress ulcers. Gastroenterology 1991;100:1515. Martin LF et al. Continuous intravenous cimetidine decreases frequency of upper GI hemorrhage without promoting pneumonia. Crit Care Med 1993;21:19. Levy MJ et al. Comparison of omeprazole and ranitidine for stress ulcer prophylaxis. Dig Dis Sci 1997;42:1255. Frank W et al. Comparison between continuous and intermittent infusion regimens of cimetidine in ulcer patients. Clin Pharmacol Ther 1989;46:234. Siepler JK et al. Use of continuous infusion of histamine2-receptor antagonists in critically ill patients. DICP 1989;23:S40. Tryba M. Risk of acute stress bleeding and nosocomial pneumonia in ventilated intensive care unit patients: sucralfate versus antacids. Am J Med 1987;83(Suppl 3B):117. Bresalier RS et al. Sucralfate suspension versus titrated antacid for the prevention of acute stressrelated gastrointestinal hemorrhage in critically ill patients. Am J Med 1987;83(Suppl 3B):110. Cook D et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med 1998;338:791.