3 - Montclair State University
advertisement

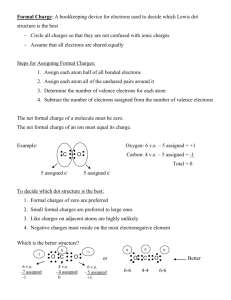
ADVANCED INORGANIC CHEMISTRY October 19, 2009 INSTRUCTIONS: PRINT YOUR NAME ————> NAME Quiz 3 ___________________ . WORK 1 & 2 – WORK 2 of 3-5 TOTAL = 100 SHOW YOUR WORK FOR PARTIAL CREDIT USE THE CORRECT NUMBER OF SIGNIFICANT FIGURES THE LAST PAGE IS A PERIODIC TABLE R = 0.08206 lit-atm/mol-K R = 8.3145 J/mol-K 1 2 h = 6.626 X 10-34 J-s 3 25 c = 2.9979 X 108 m/s 4 25 5 25 J = (kg-m2)/s2 TOTAL(100) 25 25 1. Draw a valid Lewis structure for the following molecules or ions. Include formal charges to each atom in (b), (d), and (e). (The central atom is listed first except for (f)) (a) NOCl (b) ICl4+ (d) ClOF4- (e) I3- (c) SO3 (f) N2Cl4 (symmertic with an N-N bond) 2. Draw a valid Lewis structure and determine shape of the following molecules using VSEPR. Assign formal charges in (b) and (f). For neutral species (a), (c), (d), and (e), assume all bonds in are polar, and determine whether the molecule is polar. (The central atom is listed first in each formula.) (a) SCl2 (b) SBr3- (d) XeBr4 (e) XeO3 (c)COCl2 (f)SO22- Polar or non-polar (a) SCl2____________________________(c)COCl2_______________________________ (d) XeBr4________________________________________ (e) XeO3__________________________________________ 3. Consider the chlorate ion, ClO3(a) Draw a valid Lewis structure for this ion that obeys the octet rule and assign formal charges to each atom. (b) Draw a valid Lewis structure for this ion that has more reasonable formal charges and assign formal charges to each atom. (c) Draw the resonance structures for the structure that you drew in (b). (d) What shape is the chlorite ion? . 4. Phosphorus pentachloride exist as isolated molecules in the gaseous and liquid states. In the solid state it crystallizes as an ionic solid. Both the cation and anion contain phosphorus. (a) Draw the structure of the molecular form of this compound, and describe its shape. (b) Draw the structure of the cation and anion found in the solid state, assign formal charges to the atoms, and describe the shape of each species. 5. Cyanate, OCN-, and fulminate, ONC-, are isomeric ions. Cyanates are stable compounds. Fulminates are unstable and are used in the preparation of detonation caps for explosives. (a) Draw three resonance structures of the cyanate ion and assign formal charges to each atom. (a) Draw three resonance structures of the fulminate ions and assign formal charges to each atom. (c) Based on the structures that you drew above, explain why cyanate is a more stable ion than fulminate.