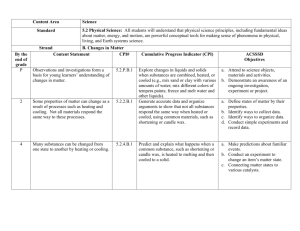
Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS
advertisement

Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS ? Day 1 Learning Objectives: Understand Units of measurements, uncertainty of measurements, significant figures and calculations, dimensional analysis. Define and evaluate the uncertainty, accuracy, and precision of measurements Perform mathematical computations using methods of numerical problem solving. NJCCC standards addressed: 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will review chapters 1, 2 & 3 Students will take turns in solving problems on the board as the class corrects their summer assignment Summary/Assessment: Homework: Review chapter 1,2 &3 notes Check answers to the summer assignment Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS?( chapter 1,2 &3) Day2 Learning Objectives: Students will be able to: Find the Atomic mass of a metal by converting a weighed amount of a unknown Metal to an Oxide Determining formula of a compound, using stoichiometric calculations Apply the law of Dulong and Petite to determine the Atomic weight of a Unknown Metal NJCCC standards addressed: 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS HOW CAN THE ATOMIC MASS OF AN UNKNOWN ELEMENT BE DETERMINED Development: Students will perform the procedure of the Atomic Weight of a Metal lab Students will use their collect data to calculate the ratio of the mass of oxygen that combined with 1 gram of the metal and predict a possible empirical formula of the metal Oxide and the identity of the metal Students will apply the law of Dulong and Petite to determine the empirical formula of the metal Oxide and the identity of the metal Summary/Assessment: Compare Class results Discuss the importance of the law of Dulong and Petite in determing the atomic weight of some metallic elements Homework: Lab report for Atomic Weight of a Metal lab due Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS?( chapter 1,2 &3) Day3 Learning Objectives: Students will be able to: Find the Atomic mass of a metal by converting a weighed amount of a unknown Metal to an Oxide Determining formula of a compound, using stoichiometric calculations Apply the law of Dulong and Petite to determine the Atomic weight of a Unknown Metal NJCCC standards addressed: 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS HOW CAN THE ATOMIC MASS OF AN UNKNOWN ELEMENT BE DETERMINED Development: Students will perform the procedure of the Atomic Weight of a Metal lab Students will use their collect data to calculate the ratio of the mass of oxygen that combined with 1 gram of the metal and predict a possible empirical formula of the metal Oxide and the identity of the metal Students will apply the law of Dulong and Petite to determine the empirical formula of the metal Oxide and the identity of the metal Summary/Assessment: Compare Class results Discuss the importance of the law of Dulong and Petite in determing the atomic weight of some metallic elements Homework: Lab report for Atomic Weight of a Metal lab due Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS (Chapter 1, 2 &3) Day4 Learning Objectives: Students will be able to: Write and name formulas of ionic compounds. Write and name formulas of ionic compounds using ions with more than one oxidation # Apply the rules for naming and writing the formulas of acids Write and name formulas of Binary Molecular Compound. NJCCC standards addressed: 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will review chapters 1, 2 & 3 Students will take turns in solving problems on the board as the class corrects their summer assignment Summary/Assessment: Homework Review chapter 1,2 &3 Homework worksheets Chapter 1,2,3 Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS( Chapter 1,2 &3) Day5 Learning Objectives: Students will be able to: Find the percent composition of a compound Determining formula of a compound, using stoichiometric calculations, Find the amounts of reactants and products Perform calculations involving limiting reactants. NJCCC standards addressed: 55.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will review chapters 1, 2 & 3 Students will take turns in solving problems on the board as the class corrects their summer assignment Summary/Assessment: Chapter 1, 2 &3 Test Homework Review for the Test Riar: A.P Chemistry UNIT 1: REVIEW OF CHEMISTRY BASICS( Chapter 1,2 &3) Day6 Learning Objectives: Students will be able to: Find the percent composition of a compound Determining formula of a compound, using stoichiometric calculations, Find the amounts of reactants and products Perform calculations involving limiting reactants. NJCCC standards addressed: 55.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will review chapters 1, 2 & 3 Students will take turns in solving problems on the board as the class corrects their summer assignment Summary/Assessment: Chapter 1, 2 &3 Test Homework Review for the Test Riar: A.P Chemistry UNIT 2: Solutions and net ionic reactions ( chapter 4 Part I) Day1 Learning Objectives: Students will be able to: Demonstrate knowledge of solution Stoichiometry including strong, weak and non electrolytes. Calculate the Molarity of unknown solutions and ions in the solution know the different parts of a solution, how they can influence a solution’s properties Calculate the concentration of a solution. Describe how to make a solution of known concentration Describe how to make a solution of a known concentration by diluting a stock solution NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Demonstrations – Conductivity of soluble Ionic compds Electrolytes/ Non Electrolytes Weak and strong Electrolytes Development: Solution Power point lecture Summary/Assessment: Homework Section 1 – problems 5,7,11,15,17,19abef,20ace,21,22,23,24,82ad,93,95 Riar: A.P Chemistry UNIT 2: Solutions and net ionic reactions( chapter 4 Part I) Day 2 Learning Objectives: Students will be able to: Demonstrate knowledge of solution Stoichiometry including strong, weak and non electrolytes. Calculate the Molarity of unknown solutions and ions in the solution know the different parts of a solution, how they can influence a solution’s properties Calculate the concentration of a solution. Describe how to make a solution of known concentration Describe how to make a solution of a known concentration by diluting a stock solution NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solution Power point lecture Summary/Assessment: Homework Section 2- Problems 25all,27all,26all,29,30,31,32,33,34,67,41,43,87 Riar: A.P Chemistry UNIT 2: Solutions and net ionic reactions( chapter 4 Part I) Day 3 Learning Objectives: Students will be able to: Apply memorized solubility rules in writing precipitation reactions Write the Overall Ionic equations and Net Ionic equations for Double Displacement Reactions Find the amounts of reactants and products Perform calculations involving limiting reactants. NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solution Power point lecture Summary/Assessment: Homework Section 3 – Problems 35,36,37,38,39,40,68,70,71,72,73,74,83,84,85,90,91 Riar: A.P Chemistry UNIT 2: Solutions and net ionic reactions( chapter 4 Part I) Day4 Learning Objectives: Students will be able to: Perform Calculations involving acid-base titration Identify and explain the changes in precipitation, acid base reactions Understand the terms associated with acid-base reactions. NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solution Power point lecture Summary/Assessment: Homework Section 4 – Problems 45ac,46b,47,48,49,51,52,75,76,77,94 Riar: A.P Chemistry UNIT 2: Solutions and net ionic reactions( chapter 4 Part I) Day5 Learning Objectives: Students will be able to: Discover the mole ratio in which substances react and find the balancing coefficients for the reaction without knowing the identity of the reactants To plot and interpret a continuous variation graph using graphing calculator NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will perform the procedure of the continuous variation lab Students will use their collect data and given Molarity of the unknown reactants A & B to calculate the mole/mole ratio Students will use their collect data and class data to construct a continuous variation graph using a graphing calculator Students will be able to predict the mole/mole ratio of the two reactants from their graph using the linear regression equations of the two lines which intersect at the ideal mole ratio Summary/Assessment: Compare Class results Discuss the importance of the Continuous variation method of finding the stoichiometric ratios of two unknown reactants. Homework Chapter 4 Section4 - 53,55,57,59, and 61 Review chapter 4 Part I test Riar: A.P Chemistry UNIT 2: Solutions and net ionic reactions( chapter 4 Part I) Day 6 Learning Objectives: Students will be able to: Discover the mole ratio in which substances react and find the balancing coefficients for the reaction without knowing the identity of the reactants To plot and interpret a continuous variation graph using graphing calculator NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will perform the procedure of the continuous variation lab Students will use their collect data and given Molarity of the unknown reactants A & B to calculate the mole/mole ratio Students will use their collect data and class data to construct a continuous variation graph using a graphing calculator Students will be able to predict the mole/mole ratio of the two reactants from their graph using the linear regression equations of the two lines which intersect at the ideal mole ratio Summary/Assessment: Compare Class results Discuss the importance of the Continuous variation method of finding the stoichiometric ratios of two unknown reactants. Homework Review chapter 4 Part I test Riar: A.P Chemistry UNIT 2: Oxidation / Reduction Reactions – Redox Reactions ( chapter 4 Part II) Day 1 Learning Objectives: Students will be able to: Explain what is an Oxidation State Assign Oxidation # to elements in various compounds and in the elemental state Identify Oxidation – Reduction Reactions and the corresponding oxidizing and reducing agent NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Oxidation of Cu metal in HNO3 acid solution Demo Development: Redox Power point presentation Summary/Assessment: Students will be able Assign Oxidation # to elements in various compounds and in the elemental state Explain what is an Oxidizing and reducing agent Homework Worksheet assigning oxidation # to various elements Riar: A.P Chemistry UNIT 2: : Oxidation / Reduction Reactions – Redox Reactions ( chapter 4 Part II) Day 2 Learning Objectives: Students will be able to: Identify a redox reaction Identify and write Oxidation and Reduction Half reactions Balance Redox reactions by the change in oxidation number method Balance Redox reactions by Half reaction method NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Redox Power point presentation Students will follow the method for balancing the Redox reactions Summary/Assessment: Exit Ticket – Balance one Redox reaction in acid solution Homework: Chapter 4 Section4 – 63,79, 80, 81,92all Riar: A.P Chemistry UNIT 2: Oxidation / Reduction Reactions – Redox Reactions ( chapter 4 Part II) Day 3 Learning Objectives: Students will be able to: Perform a Redox Titration Standardize a Stock KMnO4 (aq) Solution Find the % of Fe+2 in Iron Pills NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will perform the procedure of Part A Standardization of KMnO4 Students will use their collected data of volume of KMnO4 used to titrate the samples to calculate the Molarity of the KMnO4solution. Students will use the moles of KMnO4 used to calculate the moles of Fe+2 present in the pill sample based on the balanced redox equation Students will determine the gram Fe+2 equivalent to this number of moles. Students will Compare Class results Summary/Assessment: Discuss the importance of the Volumetric analysis method of finding the stoichiometric ratios of reactants. Homework Lab report for the % Fe +2 in Iron pills lab due Riar: A.P Chemistry UNIT 2: : Oxidation / Reduction Reactions – Redox Reactions ( chapter 4 Part II) Day 4 Learning Objectives: Students will be able to: Identify and write Oxidation and Reduction Half reactions Balance a Redox reaction in an acid solution Balance a Redox reaction in an Basic solution Balance Redox reactions by Half reaction method NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Redox Power point presentation Students will follow the method for balancing the Redox reactions Summary/Assessment: Homework Mortimer Questions-13.13all ,13.19all ,13.21 all UNIT 2: : Oxidation / Reduction Reactions – Redox Reactions ( chapter 4 Part II) Day 5 Learning Objectives: Students will be able to: Balance a Redox reaction and do Volumetric analysis of the products Balance Redox reactions by Half reaction method Predict Products of a Redox reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will be given an worksheet with a table that shows that possible products of oxidation and reduction reaction Students will follow the example problems for predicting products Summary/Assessment: Homework A.P Exam questions an predicting Products of Redox reactions for the years 72, 73,74, 76,77, 80 , 82 ,85, 89 Riar: A.P Chemistry UNIT 2: : Oxidation / Reduction Reactions – Redox Reactions ( chapter 4 Part II) Day 6 Learning Objectives: Students will be able to: Balance a Redox reaction and do Volumetric analysis of the products Balance Redox reactions by Half reaction method Predict Products of a Redox reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will be given an worksheet with a table that shows that possible products of oxidation and reduction reaction Students will follow the example problems for predicting products Summary/Assessment: Review Chapter 4 part II Redox reactions Homework A.P Exam questions an predicting Products of Redox reactions for the years 72, 73,74, 76,77, 80 , 82 ,85, 89 Chapter 4 part II Redox reactions test Riar: A.P Chemistry UNIT 3: : Gases Chapter 5 Learning Objectives: Students will be able to: Understand what is pressure and units of measuring pressure Understand how does a barometer works? And what does it measure Convert from one unit of pressure to the other NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Show them a Barometer and how to read a barometer Demo: Crushing the can Development: Gas Laws chapter 5 Power point presentation Summary/Assessment: Homework Section 2 - 59,61, 63, 85, 97,99 Riar: A.P Chemistry UNIT 3: : Gases Chapter 5 Learning Objectives: Students will be able to: Understand the Ideal gas Law formulated by Boyle, Charles, Dalton , and Graham and solve problems based on these laws Use the Kinetic Molecular Theory to explain the theoretical basis for the gas laws NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Demo: Hydrogen and Oxygen Balloon and hydrogen Balloon Development: Gas Laws chapter 5 Power point presentation Summary/Assessment: Homework Section 1 - 21,23,25,33,41,45,57,91,93,94,98,99 Riar: A.P Chemistry UNIT 3: : Gases Chapter 5 Learning Objectives: Students will be able to: Find the density of a sample of a gas at STP if its mass, volume and pressure are known under non standard conditions Use the Kinetic Molecular Theory to explain the theoretical basis for the gas laws Find the density of a sample of a gas at specific temperature if density of the gas at another temperature and pressure is known Understand what is a mole fraction and how is it represented Understand What does Dalton’s law mean when you collect a gas over water Understand what is Partial pressure? Understand the difference between diffusion and effusion Calculate the Root mean square velocity of the gas particles under given conditions NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Gas Laws chapter 5 Power point presentation Summary/Assessment: Homework Section 3 - 47, 49, 51, 52, 53,67,68,89 Riar: A.P Chemistry UNIT 3: : Gases Chapter 5 Learning Objectives: Students will be able to: Find the Formula Mass of the disposable lighter gas using the Ideal gas Laws NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will perform the procedure of the Lighter gas lab Students will measure the volume of the lighter gas collected and measure the temperature of the gas and find the pressure of the gas collected over water from the barometric pressure Students will use their collect data and Ideal gas law to determine the formula mass of the unknown lighter gas Students will Compare Class results Summary/Assessment: Homework Lab report for the unknown lighter gas lab due Riar: A.P Chemistry UNIT 3: : Gases Chapter 5 Learning Objectives: Students will be able to: Understand the Ideal gas behavior Understand the typical conditions for non-ideal behavior Understand the Vander wails gas equation as the corrected gas equation for non-ideal conditions Understand What a and b stand for in Vander Waals equation NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Gas Laws chapter 5 Power point presentation Summary/Assessment: Homework 69,71,73,75,77,78,79,81,83,84,110,111 Test Chapter 5 Gases Riar: A.P Chemistry UNIT 3: : Gases Chapter 5 Learning Objectives: Students will be able to: Solve mixed mass volume problems when there are standard conditions of temperature and pressure Solve mixed mass volume problems when there are non standard conditions of temperature and pressure Use Avogadro’s law in stoichiometry NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Gas Laws chapter 5 Power point presentation Summary/Assessment: Homework Old A.P Exam Free Response questions Test Chapter 5 Gases Riar: A.P Chemistry UNIT 4: Thermo Chemistry Chapter 6 Learning Objectives: Students will be able to: Distinguish the various forms that energy takes in an ensemble of atoms or molecules Understand the first law of thermodynamics Understand the difference between Heat and Temperature Understand the difference between a system and surroundings Understand that the internal energy of a system is changed by the transfer of heat and work Recognize that temperature ,volume ,pressure and energy are all state functions NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Conservation of energy and transfer of energy Demo Development: Thermo Chemistry Chapter 6 power point presentation Summary/Assessment: Homework Read and summarize the National Geographic Article from Oct 2007- Global Warming Riar: A.P Chemistry UNIT 4: Thermo Chemistry Chapter 6 Learning Objectives: Students will be able to: Explain which way is heat moving and the sign of each Understand difference between Exothermic and Endothermic reactions Calculate the change in energy as a result of heat transfer or work done or both Understand the difference between specific heat and heat capacity NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Thermo Chemistry Chapter 6 power point presentation Summary/Assessment: Homework Section 1 - 31,32,33,36,39,43,45,47,48,50,75,81 Riar: A.P Chemistry UNIT 4: Thermo Chemistry Chapter 6 Learning Objectives: Students will be able to: Understand what is Hess law Understand what is enthalpy Calculate the change in enthalpy of a reaction by applying Hess law Explain the difference between the direct method and indirect method of solving for enthalpy NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Thermo Chemistry Chapter 6 power point presentation Summary/Assessment: Homework Section 2 Hess law- 53,55,57,59,61,63,65,67,78,81,82,83,88,89 Riar: A.P Chemistry UNIT 4: Thermo Chemistry Chapter 6 Learning Objectives: Students will be able to: Measure the heat of the reaction associated with complete ionization of Ammonia in water Measure the enthalpy change of two separate reactions and then apply Hess law to calculate the overall enthalpy change of the desired reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Students will perform the procedure of the Enthalpy change for the complete Ionization of ammonia lab Students will use their collect data to calculate the heat transferred in both the reactions and then calculate the H for both reactions Students will apply Hess law to calculate the H of the desired reaction Students will compare and analyze class results Summary/Assessment: Homework Lab report Enthalpy change for the complete Ionization of ammonia lab due Riar: A.P Chemistry UNIT 4: Thermo Chemistry Chapter 6 Learning Objectives: Students will be able to: Understand what is the Bond energy Calculate the H by the Bond energy equation Understand what is lattice energy Explain the effect of ionic radius and charge of the ion on the Lattice energy of an Ionic Compound Illustrate the formation of an Ionic solid and calculate the H of formation by Bohrn Haber Cycle NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Thermo Chemistry Chapter 6 power point presentation Summary/Assessment: Homework Section 2 Chapter 8-35,37,39,41,43,45,55,51 A.P Free Response questions Riar: A.P Chemistry UNIT 4: Thermo Chemistry Chapter 6 Learning Objectives: Students will be able to: Understand what is the Bond energy Calculate the H by the Bond energy equation Understand what is lattice energy Explain the effect of ionic radius and charge of the ion on the Lattice energy of an Ionic Compound Illustrate the formation of an Ionic solid and calculate the H of formation by Bohrn Haber Cycle NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Thermo Chemistry Chapter 6 power point presentation Summary/Assessment: Homework Review for the test Thermo Chemistry Chapter 6 Test Riar: A.P Chemistry UNIT 5: Atomic Structure and Periodicity Chapter 7 Learning Objectives: Students will be able to: Explain what is Electromagnetic Radiation Explain the relationship between the wavelength and frequency of high energy gamma rays and low energy radio waves Understand the wave particle duality nature of light NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Atomic Structure and Periodicity Chapter 7 power point presentation Summary/Assessment: Homework Section 1 - 35,37, 41, 47,49,54,109,110,111 Riar: A.P Chemistry UNIT 5: Atomic Structure and Periodicity Chapter 7 Learning Objectives: Students will be able to: Explain the basis of quantum theory Understand the atomic spectra of Hydrogen and Bohr’s model of an atom Understand what are photons and emission spectra Understand the difference between an electron in a ground state and excitedstate Calculate the energy of an electron in its ground state and energy needed to move to a higher energy state NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Atomic Structure and Periodicity Chapter 7 power point Presentation Summary/Assessment: Homework Section 2- 49,51,109,110,111,113,114, 131 Riar: A.P Chemistry UNIT 5: Atomic Structure and Periodicity Chapter 7 Learning Objectives: Students will be able to: Explain the use of wave mechanics in atomic theory Describe the wave -mechanical model of an atom Understand the Heisenberg’s uncertainty Principle Understand the 4 quantum #s, orbital shapes, electron spin and Pauli’s exclusion Principle Explain how each of the quantum #s indicate an Electron location NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Atomic Structure and Periodicity Chapter 7 power point presentation Summary/Assessment: Homework Section 2 44,55,57,59,60,63,64,65,66,67,71,73,74,75,77,78,79,80,113,114,115,117,119,121,129, Riar: A.P Chemistry UNIT 5: Atomic Structure and Periodicity Chapter 7 Learning Objectives: Students will be able to: Understand what is an electron Configuration Write an electron configuration of an element Explain Hund’s Rule and Aufbau principle Explain the relationship between valence electrons and columns Understand what is an atomic radius and the trends in the P.T Explain how are ions derived from representative elements Explain the concept of shielding NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Atomic Structure and Periodicity Chapter 7 power point presentation Summary/Assessment: Homework Section 3 81,83,85,87,89,90,91,93,95,97,99,101,123,124,125,126,128,133,134 A.P Exam Free Response Take home due Riar: A.P Chemistry UNIT 5: Atomic Structure and Periodicity Chapter 7 Learning Objectives: Students will be able to: Understand the history of the Periodic table and the contributions of Moseley and Mendeleev Explain the relationship between valence electrons and columns Understand what is an atomic radius and the trends in the P.T Explain how are ions derived from representative elements Understand what is Ionization energy and the trends in the P.T Understand what is Electron affinity energy and the trends in the P.T Understand what is an Ionic radius and the trends in the P.T Explain how the properties of a group - Alkali metals NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Atomic Structure and Periodicity Chapter 7 power point presentation Summary/Assessment: Homework Section 3 81,83,85,87,89,90,91,93,95,97,99,101,123,124,125,126,128,133,134 A.P Exam Free Response Take home due Riar: A.P Chemistry UNIT 5: Atomic Structure and Periodicity Chapter 7 Learning Objectives: Students will be able to: Understand the history of the Periodic table and the contributions of Moseley and Mendeleev Explain the relationship between valence electrons and columns Understand what is an atomic radius and the trends in the P.T Explain how are ions derived from representative elements Understand what is Ionization energy and the trends in the P.T Understand what is Electron affinity energy and the trends in the P.T Understand what is an Ionic radius and the trends in the P.T Explain how the properties of a group - Alkali metals NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Atomic Structure and Periodicity Chapter 7 power point presentation Summary/Assessment: Homework Review for Atomic Structure and Periodicity Chapter 7 Riar: A.P Chemistry UNIT 6: Chemical Bonding Chapter 8 &9 &10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Lewis electron-dot symbol, Lewis electron-dot structure, octet rule ionic bonding, covalent bonding, coordinate covalent bond ,bond order ,bond type, bond length Polar covalent bond, partial positive charge partial negative charge, non polar covalent bond Give the Lewis electron-dot symbol for elements Give the Lewis electron-dot symbols for ions Drawing Lewis electron-dot structures for molecules and polyatomic ion Go into detail about ionic bonding Use Lewis electron-dot structures to describe ionic bonding Go into detail about covalent bonding Use Lewis electron-dot structures to describe covalent bonding Identify bond order Draw Lewis structures for molecules that do not obey the octet rule Classify bond types according to electronegativity NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Chemical Bonding Chapter 8 power point presentation Summary/Assessment: Homework 15,17,21,23,25(a,b) ,27, 31(a,b,c) 33,35 ,37, 39 Riar: A.P Chemistry UNIT 6: Chemical Bonding Chapter 8 &9 &10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Lewis electron-dot symbol, Lewis electron-dot structure, octet rule, ionic bonding covalent bonding, coordinate covalent bond,bond order ,bond type, bond length Polar covalent bond, partial positive charge partial negative charge, non polar covalent bond Give the Lewis electron-dot symbol for elements Give the Lewis electron-dot symbols for ions Drawing Lewis electron-dot structures for molecules and polyatomic ion Go into detail about ionic bonding Use Lewis electron-dot structures to describe ionic bonding Go into detail about covalent bonding Use Lewis electron-dot structures to describe covalent bonding Identify bond order Draw Lewis structures for molecules that do not obey the octet rule Classify bond types according to electronegativity NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Chemical Bonding Chapter 8 power point presentation Summary/Assessment: Homework 41,91,92,93,107 ,55,95 ,96(ef), 105 Riar: A.P Chemistry UNIT 6:Chemical Bonding Chapter 8&9&10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: delocalized bonding ,resonance description, resonance bonding ,resonance forms , polar covalent bond moderately polar covalent bond ,very polar covalent bond , polar molecule, formal charge Identify delocalized bonding Draw resonance structures Draw Lewis structures for molecules that do not obey the octet rule Classify bond types according to electronegativity Calculate formal charges Determine which Lewis electron-dot structure is best if more than one is possible NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Chemical Bonding Chapter 8 power point presentation Summary/Assessment: Homework 57 all,58 all,59,61,63,66,67,68,69,70,71&72(fgh),73,75,98,99 Riar: A.P Chemistry UNIT 6:Chemical Bonding Chapter 8&9&10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Molecular geometry, Valence-Shell-Electron-Pair-Repulsion (VSEPR) Model, single bonds, multiple bond ,double bonds, triple bond , lone pairs non-bonding pairs, dipole moment, valence bond theory overlapping , hybridization, sigma bond , pi bond Go into detail about the Valence-Shell-Electron-Pair-Repulsion (VSEPR) Model Go into details about the rules of thumb for electron pair repulsion Explain why multiple bonds take up more "space" than single bonds Apply the three rules to minimize electron pair repulsion in a structure Predict and describe the geometry of compounds with two, three, or four pairs of electrons on the central atom, draw the most correct structure for them, and explain why that structure is the most correct using the VSEPR model Predict and describe the geometry of compounds with five or six pairs of electrons on the central atom, draw the most correct structure for them, and explain why that structure is the most correct using the VSEPR model NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Chemical Bonding Chapter 9 power point presentation Summary/Assessment: Homework 73,77&8 ,79&83,85(cd) ,87,89,100,103,109,111,112(c),113 Riar: A.P Chemistry UNIT 6:Chemical Bonding Chapter 8&9&10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Molecular geometry , Valence-Shell-Electron-Pair-Repulsion (VSEPR) Model, single bonds, multiple bond ,double bonds, triple bond , lone pairs non-bonding pairs, dipole moment, valence bond theory overlapping , hybridization, sigma bond , pi bond Go into detail about the Valence-Shell-Electron-Pair-Repulsion (VSEPR) Model Go into details about the rules of thumb for electron pair repulsion Explain why multiple bonds take up more "space" than single bonds Apply the three rules to minimize electron pair repulsion in a structure Predict and describe the geometry of compounds with two, three, or four pairs of electrons on the central atom, draw the most correct structure for them, and explain why that structure is the most correct using the VSEPR model Predict and describe the geometry of compounds with five or six pairs of electrons on the central atom, draw the most correct structure for them, and explain why that structure is the most correct using the VSEPR model Describe the relationship between dipole moment and a bond’s polarity Relate dipole moment and molecular geometry for binary molecular compounds and predict whether or not a binary molecular compound would be expected to have a zero net dipole moment on the basis of its geometry NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Chemical Bonding Chapter 9 power point presentation Summary/Assessment: Homework: 9,11,13,23(fgjkm)25,26,27,28,29,51,53,54(ab),61 Riar: A.P Chemistry UNIT 6: Chemical Bonding Chapter 8&9&10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Molecular geometry , Valence-Shell-Electron-Pair-Repulsion (VSEPR) Model, single bonds, multiple bond ,double bonds, triple bond , lone pairs non-bonding pairs, dipole moment, valence bond theory overlapping , hybridization, sigma bond , pi bond Describe the relationship between dipole moment and a bond’s polarity Relate dipole moment and molecular geometry for binary molecular compounds and predict whether or not a binary molecular compound would be expected to have a zero net dipole moment on the basis of its geometry Go into detail about valence bond theory Go into detail about how promotion and hybridization explain bond formation and bond strength Go into detail using valence bond theory to describe multiple bonding Predict the hybridization in a compound or bond NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Chemical Bonding Chapter 9 power point presentation Summary/Assessment: Homework 29, 31, 33, 35, 85,98 Riar: A.P Chemistry UNIT 7: Solutions Chapter 10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Be able to explain why ionic interactions, hydrogen bonds, and dipole-dipole interactions are electrostatic interactions Be able to describe, compare and contrast the types of intermolecular forces Be able to describe the two factors that affect dispersion forces and be able to predict relative strength of dispersion forces for substances from their formula and/or structure Be able to describe hydrogen bonds and be able to predict whether or not hydrogen bonds will exist and the relative strength of hydrogen bonding for substances from their formula and/or structure Be able to identify which intermolecular forces are present in substance from their formula and/or structure Explain the relationship between intermolecular forces and the five mentioned properties of liquids Be able to describe how the structure of each of the four types of solids explains their physical properties NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Liquids and Solids Chapter 10 power point presentation Summary/Assessment: Homework Homework 29, 31, 33, 35, 85,98, 83,75,77,79,84 Riar: A.P Chemistry UNIT 7: Solutions Chapter 10 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: heat of vaporization ,vapor pressure, boiling point ,melting, freezing, vaporization condensation, sublimation, deposition ,heat of fusion – standard enthalpy of fusion, heat of vaporization – standard enthalpy of vaporization, volatility, equilibrium vapor pressure, Clausius-Clapeyron equation, phase diagram ,triple point, critical point, critical temperature ,critical pressure , Heating curve NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Liquids and Solids Chapter 10 power point presentation Summary/Assessment: Homework Review for Part-I Chemical Bonding Chapter 8,9&10 Test Part-I Chemical Bonding Chapter 8,9 &10 Test Take home Part-II Chemical Bonding Chapter 8, 9&10 Test due Riar: A.P Chemistry UNIT 7: Solutions Chapter 11 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Solution, crystallization, soluble, insoluble, miscible , immiscible, solubility, saturated solution, unsaturated solution, supersaturated solution, molarity, mass percentage of solute, molality, mole fraction, Henry’s Law Compare and contrast, and give examples of the three types of solutions based on state Describe the solution process in terms of free energy Describe the solution process in terms of the three processes involved in the formation of a solution Describe the solution process in terms of enthalpy changes, entropy changes, whether a solution will form or not, and whether the solution process will be exothermic or endothermic Explain the application of enthalpy changes and entropy changes to the rule, “Like dissolves like.” Describe the effects of temperature and pressure on solubility and apply that to the solubility of solids and gases NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solutions Chapter 11 power point presentation Summary/Assessment: Homework 27,29, 30 (a-d),31,32,79 Riar: A.P Chemistry UNIT 7: Solutions Chapter 11 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: Solution, crystallization, soluble, insoluble, miscible , immiscible, solubility, saturated solution, unsaturated solution, supers Work Henry’s Law problems Define molarity and describe how to make a solution of a given molarity Define mass percentage of solute and describe how to make a solution of a given mass percentage of solute Define molality and describe how to make a solution of a given molality Define mole fraction and describe how to make a solution of a given mole fraction Convert percent to mole fraction Convert molality and mole fraction Convert molality and molarity Saturated solution, molarity, mass percentage of solute, molality, mole fraction, Henry’s Law NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solutions Chapter 11 power point presentation Summary/Assessment: Homework 27,29, 30 (a-d),31,32,79, 35,38,39,41,43 Riar: A.P Chemistry UNIT 7: Solutions Chapter 10& 11 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: colligative property, vapor pressure lowering, Raoult’s Law, boiling point elevation, freezing point depression, osmosis, osmotic pressure ,van’t Hoff factor Do vapor pressure lowering and Raoult’s Law problems Do boiling point elevation problems Do freezing point depression problems Use freezing point depression to determine molecular weight and molecular formula Do osmotic pressure problems Work colligative property problems for ionic solutions NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solutions Chapter 11 power point presentation Summary/Assessment: Homework 47,49,51,55,56,57,58 Riar: A.P Chemistry UNIT 7: Solutions Chapter 11 Learning Objectives: Students will be able to: Determine Freezing temperature of a pure solvent, Lauric Acid Determine Freezing temperature of a mixture of lauric Acid and benzoic acid Calculate the freezing point depression of the mixture Calculate the molecular weight of benzoic acid. NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS HOW CAN THE MOLECULAR WEIGHT OF AN UNKNOWN SOLUTE BE DETERMINED BY USING FREEZING POINT DEPRESSION Development: Students will perform the procedure of the Using freezing point depression to find molecular weight lab Students will use their collect data to calculate the molecular mass of the unknown solute Students will plot a graph of their collected data to determine the freezing temperature of a mixture of lauric Acid and benzoic acid Students will calculate the freezing point depression of the mixture Summary/Assessment: Discuss Lab results and compare class results Homework Complete lab write up due Riar: A.P Chemistry UNIT 7: Solutions Chapter 11 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: colligative property, vapor pressure lowering, Raoult’s Law, boiling point elevation, freezing point depression, osmosis, osmotic pressure ,van’t Hoff factor Do vapor pressure lowering and Raoult’s Law problems Do boiling point elevation problems Do freezing point depression problems Use freezing point depression to determine molecular weight and molecular formula Do osmotic pressure problems Work colligative property problems for ionic solutions NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solutions Chapter 11 power point presentation Summary/Assessment: Homework 59,61,62,63,64,65,66,71,72,73,85 Riar: A.P Chemistry UNIT 7: Solutions Chapter 11 Learning Objectives: Students will be able to: Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: colligative property, vapor pressure lowering, Raoult’s Law, boiling point elevation, freezing point depression, osmosis, osmotic pressure ,van’t Hoff factor Do vapor pressure lowering and Raoult’s Law problems Do boiling point elevation problems Do freezing point depression problems Use freezing point depression to determine molecular weight and molecular formula Do osmotic pressure problems Work colligative property problems for ionic solutions NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Solutions Chapter 11 power point presentation Summary/Assessment: Homework Take Home solutions test due Review for Solutions Test Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define chemical kinetics, reaction rate, average rate, instantaneous rate, rate constant, rate law, reaction order Analyze and apply the factors that affect reaction rate Relate the rate of disappearance of a reactant and the rate of appearance of a product from the balanced equation Determine the average reaction rate from concentration measurements at specific times Give the reaction order with respect to each reactant and give the overall reaction order for a reaction when given its rate law Determine the rate law from initial concentrations and initial rates NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Section 1 – 19, 20, 21, 22, 23, 25, 27, 63, 71 Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define catalyst, inhibitor, homogeneous catalysis, heterogeneous catalysis, enzyme catalysis, active site ,substrate Analyze and apply the factors that affect reaction rate Relate the rate of disappearance of a reactant and the rate of appearance of a product from the balanced equation Determine the average reaction rate from concentration measurements at specific times Give the reaction order with respect to each reactant and give the overall reaction order for a reaction when given its rate law Determine the rate law from initial concentrations and initial rates NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Section 1 – 19, 20, 21, 22, 23, 25, 27, 63, 71 Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define chemical kinetics, reaction rate, average rate, instantaneous rate, rate constant, rate law, reaction order, half life Work rate problems using concentration-time laws for both first order and second order reactions Calculate half-lives for both first order and second order reactions Use graphing to determine the order of a reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Section 2 – 29,30,31,32,33,35,37,38,39,41,43,65,73 Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define chemical kinetics, reaction rate, average rate, instantaneous rate, rate constant, rate law, reaction order, half life Work rate problems using concentration-time laws for both first order and second order reactions Calculate half-lives for both first order and second order reactions Use graphing to determine the order of a reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Section 2 – 29,30,31,32,33,35,37,38,39,41,43,65,73 Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define -reaction mechanism, elementary step, reaction intermediate molecularity, unimolecular, bimolecular, termolecular Describe reaction mechanisms in terms of elementary steps, reaction intermediates, and molecularity Write the overall balanced equation from the mechanism Write rate laws for elementary steps Write rate laws from mechanisms Determine the rate-determining step from the rate law NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Section 3 – 45,47,48, 68, 69, 74 Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define - collision theory, activation energy ,activated complex collision theory, Arrhenius equation Use collision theory and energy considerations to explain reactions and reaction rates Explain reactions and reaction rates using potential energy profiles, including activation energy and the activated complex Use collision theory and energy considerations to explain the effect of temperature, surface area, concentration, and the presence or absence of a catalyst on reaction rates Use the Arrhenius equation to describe the relationship among activation energy, temperature, frequency of collisions, and the rate law constant and to calculate these values Describe the nature of a catalyst and catalysis, including enzyme catalysis NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Review Kinetics Kinetics take home test due Riar: A.P Chemistry UNIT 8: Kinetics Chapter 12 Learning Objectives: Students will be able to: Define chemical kinetics, reaction rate, average rate, instantaneous rate, rate constant, rate law, reaction order Analyze and apply the factors that affect reaction rate Relate the rate of disappearance of a reactant and the rate of appearance of a product from the balanced equation Determine the average reaction rate from concentration measurements at specific times Give the reaction order with respect to each reactant and give the overall reaction order for a reaction when given its rate law Determine the rate law from initial concentrations and initial rates NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS –WHAT ARE SPONTANEOUS REACTIONS? HOW DO WE MEASURE THE RATE OF A REACTION? Development: Kinetics Chapter 12 Power point Summary/Assessment: Homework Kinetics take home test due Kinetics Test Midterm Review Riar: A.P Chemistry UNIT 9: Equilibrium Chapter 13 Learning Objectives: Students will be able to: Define reversible reaction, forward reaction, reverse reaction, chemical equilibrium, dynamic equilibrium, equilibrium position Use the equilibrium position of a reaction to determine whether products or reactants are favored in a reversible reaction Write equilibrium constant expressions for homogeneous equilibria, in terms of partial pressures where all species are gaseous, and for heterogeneous equilibria Calculate equilibrium constants from equilibrium concentrations Calculate equilibrium constants from stoichiometry NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS STATE OF EQUILIBRIUM? WHAT IS DYNAMIC EQUILIBRIUM? Development: Equilibrium Chapter 13 Power point Summary/Assessment: Homework Section 1 – 19,21,23,25,27,29,32,33,35,67 Riar: A.P Chemistry UNIT 9: Equilibrium Chapter 13 Learning Objectives: Students will be able to: Define I.C.E. (Initial-Change-Equilibrium) table, equilibrium constant (Kc), homogeneous equilibrium, heterogeneous equilibrium, reaction quotient (Qc),LeChatelier’s Principle ,catalyst Use the equilibrium position of a reaction to determine whether products or reactants are favored in a reversible reaction Apply stoichiometry to equilibrium mixtures Set up an I.C.E. (Initial-Change-Equilibrium) table Use the magnitude of the equilibrium constant to determine whether the equilibrium lies to the right or left, and whether products or reactants are favored Calculate equilibrium constants from stoichiometry Predict the direction of a reaction from the reaction quotient Calculate one equilibrium concentration given the others and the equilibrium constant Calculate equilibrium concentrations from initial concentrations and the equilibrium constant NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS STATE OF EQUILIBRIUM? WHAT IS DYNAMIC EQUILIBRIUM? Development: Equilibrium Chapter 13 Power point Summary/Assessment: Homework Section 2 – 37,39,40,41,43,45,47,51,53,55,56,57,68,69,71,72,73,77,79,80,81,82,83 Riar: A.P Chemistry UNIT 9: Equilibrium Chapter 13 Learning Objectives: Students will be able to: Define I.C.E. (Initial-Change-Equilibrium) table, equilibrium constant (Kc), homogeneous equilibrium, heterogeneous equilibrium, reaction quotient (Qc),LeChatelier’s Principle ,catalyst Use the equilibrium position of a reaction to determine whether products or reactants are favored in a reversible reaction Apply stoichiometry to equilibrium mixtures Set up an I.C.E. (Initial-Change-Equilibrium) table Use the magnitude of the equilibrium constant to determine whether the equilibrium lies to the right or left, and whether products or reactants are favored Calculate equilibrium constants from stoichiometry Predict the direction of a reaction from the reaction quotient Calculate one equilibrium concentration given the others and the equilibrium constant Calculate equilibrium concentrations from initial concentrations and the equilibrium constant NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS STATE OF EQUILIBRIUM? WHAT IS DYNAMIC EQUILIBRIUM? Development: Equilibrium Chapter 13 Power point Summary/Assessment: Homework Section 2 – 37,39,40,41,43,45,47,51,53,55,56,57,68,69,71,72,73,77,79,80,81,82,83 Riar: A.P Chemistry UNIT 9: Equilibrium Chapter 13 Learning Objectives: Students will be able to: Define I.C.E. (Initial-Change-Equilibrium) table, equilibrium constant (Kc), homogeneous equilibrium, heterogeneous equilibrium, reaction quotient (Qc),LeChatelier’s Principle ,catalyst Use the equilibrium position of a reaction to determine whether products or reactants are favored in a reversible reaction Apply stoichiometry to equilibrium mixtures Set up an I.C.E. (Initial-Change-Equilibrium) table Use the magnitude of the equilibrium constant to determine whether the equilibrium lies to the right or left, and whether products or reactants are favored Calculate equilibrium constants from stoichiometry Predict the direction of a reaction from the reaction quotient Calculate one equilibrium concentration given the others and the equilibrium constant Calculate equilibrium concentrations from initial concentrations and the equilibrium constant NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS STATE OF EQUILIBRIUM? WHAT IS DYNAMIC EQUILIBRIUM? Development: Equilibrium Chapter 13 Power point Summary/Assessment: Homework Section 2 – 37,39,40,41,43,45,47,51,53,55,56,57,68,69,71,72,73,77,79,80,81,82,83 Riar: A.P Chemistry UNIT 9: Equilibrium Chapter 13 Learning Objectives: Students will be able to: Apply LeChatelier’s Principle to changes in concentration, changes in temperature, and changes in pressure Explain how catalysts work and their effect on activation energy, rate of reaction, the composition of a reaction mixture, the value of the equilibrium constant, and the rate at which equilibrium is reached NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS STATE OF EQUILIBRIUM? WHAT IS DYNAMIC EQUILIBRIUM? Development: Equilibrium Chapter 13 Power point Summary/Assessment: Homework Section 3 – 59,61,63,65,66,75,74 Additional Problems from old A.P Exams Test Equilibrium Riar: A.P Chemistry UNIT 10: Acids & Bases Chapter 14 Learning Objectives: Students will be able to: Define acid, base ,Arrhenius acid, Arrhenius base, strong acid, weak base, Brønstead-Lowry acid, Brønstead-Lowry base, conjugate acid, conjugate base, Lewis acid, Lewis base, autoionization, pH, pOH Identify the properties of acids and bases Identify strong acids and strong bases Describe the unique quality of the hydrogen ion in water that makes it different from all other aqueous cations Discuss the evidence for the Arrhenius theory Discuss the drawbacks to the Arrhenius theory, how the Brønstead-Lowry concept developed as a result of these drawbacks Write the formulas for conjugate acids and conjugate bases Identify conjugate acids and bases NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE ACIDS AND BASES WHAT ARE SOME PROPERTIES OF ACIDS AND BASES? Development: Acids And Bases Chapter 14 Power point Summary/Assessment Homework Section 2 – 25, 26 , 27, 29,31, 32, 33, 34, 13 Riar: A.P Chemistry UNIT 10: Acids & Bases Chapter 14 Learning Objectives: Students will be able to: Define acid, base ,Arrhenius acid, Arrhenius base, strong acid, weak base, Brønstead-Lowry acid, Brønstead-Lowry base, conjugate acid, conjugate base, Lewis acid, Lewis base, autoionization, ion-product constant for water pH, pOH Give the relationship between [H+] and Arrhenius definitions of acids and bases Calculate the concentrations of H+ and OH for aqueous solutions of strong acids or strong bases Give the relationship between pH and pOH and Arrhenius definitions of acids and bases Calculate pH and pOH from molar concentrations Calculate [H+] and [OH] from pH and pOH NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE ACIDS AND BASES? WHAT ARE SOME PROPERTIES OF ACIDS AND BASES? Development: Acids And Bases Chapter 14 Power point Summary/Assessment: Homework Section 3– 35,36,37,38,40,41,43,45,47,49,50,51 Riar: A.P Chemistry UNIT 10: Acids & Bases Chapter 14 Learning Objectives: Students will be able to: Define acid, base ,Arrhenius acid, Arrhenius base, strong acid, weak base, Brønstead-Lowry acid, Brønstead-Lowry base, conjugate acid, conjugate base, Lewis acid, Lewis base, autoionization, ion-product constant for water pH, pOH Use the approximation method for calculating the concentrations of species in a weak acid solution using Ka Use the quadratic formula to calculate the concentrations of species in a weak acid solution using Ka Calculate Ka using pH Calculate percent ionization for a weak acid given the initial concentration and the pH or given the initial concentration and the Ka Calculate pH of weak acids from their % dissociation Understand the table of relative strengths of acids and Bases NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE ACIDS AND BASES? WHAT ARE SOME PROPERTIES OF ACIDS AND BASES? Development: Acids And Bases Chapter 14 Power point Summary/Assessment: Homework Section 4– 53,55,57,58,59,61,63,65,66,67,69,71,73,74,131,144,145 Riar: A.P Chemistry UNIT 10: Acids & Bases Chapter 14 Learning Objectives: Students will be able to: Define acid, base ,Arrhenius acid, Arrhenius base, strong acid, weak base, Brønstead-Lowry acid, Brønstead-Lowry base, conjugate acid, conjugate base, Lewis acid, Lewis base, autoionization, ion-product constant for water pH, pOH Calculate pH of strong Bases Use the approximation method for calculating the concentrations of species in a weak base solution using Kb Use the quadratic formula to calculate the concentrations of species in a weak base solution using Kb Use the relative strengths of acids and bases to predict whether reactants or products are favored for a reaction Calculate the Kb for the conjugate base of an acid NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE ACIDS AND BASES? WHAT ARE SOME PROPERTIES OF ACIDS AND BASES? Development: Acids And Bases Chapter 14 Power point Summary/Assessment: Homework Section 5 – 75,76,77,78,79,80,81,83,84,85,87,89,95 Riar: A.P Chemistry UNIT 10: Acids & Bases Chapter 14 Learning Objectives: Students will be able to: Define acid ionization constant – Ka, percent ionization, base ionization constant – Kb, conjugate acid, conjugate base, salt hydrolysis Calculate [H+] and pH for polyprotic acids Use bond strength and bond polarity to explain the trend in acid strength for binary acids Use electronegativity and the number of O atoms bonded to the central atom to explain the trend in acid strength for oxyacids Explain the trend in acid strength for polyprotic acids and their corresponding acid anions Use salt hydrolysis to predict whether a salt solution will be acidic, basic, or neutral Calculate the pH of salt solutions Explain the common ion effect Perform calculations involving the common ion effect NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE ACIDS AND BASES? WHAT ARE SOME PROPERTIES OF ACIDS AND BASES? Development: Acids And Bases Chapter 14 Power point Summary/Assessment: Homework A.P Exam Acid / Base Free response questions TEST ACIDS AND BASES Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15PartI Learning Objectives: Students will be able to: Define common ion effect, buffer (or buffered solution) ,Henderson-Hasselbalch equation, acid-base titration, equivalence point, end point, indicator, titration curve Explain the common ion effect Perform calculations involving the common ion effect Perform calculations involving the common ion effect Describe how to make a buffer Explain how a buffer works Identify solutions that are buffers and those that are not Derive the Henderson-Hasselbalch equation Calculate the pH of the initial buffer solution Calculate the pH of a buffer solution after the addition of acid or base to that solution Perform the calculations for making a buffer solution of a specified pH NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS A COMMON ION? WHAT ARE BUFFERS? Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Section 1- 21,22,23,25,27,29,31,33,35,37 Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15PartI Learning Objectives: Students will be able to: Define common ion effect, buffer (or buffered solution) ,Henderson-Hasselbalch equation, acid-base titration, equivalence point, end point, indicator, titration curve Explain the common ion effect Perform calculations involving the common ion effect Perform calculations involving the common ion effect Describe how to make a buffer Explain how a buffer works Identify solutions that are buffers and those that are not Derive the Henderson-Hasselbalch equation Calculate the pH of the initial buffer solution Calculate the pH of a buffer solution after the addition of acid or base to that solution Perform the calculations for making a buffer solution of a specified pH NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE BUFFERS? Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Section 2- 41,43,45,46,47,48,50,51,52,53,54,113,114,115,116 Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15PartI Learning Objectives: Students will be able to: Define common ion effect, buffer (or buffered solution) ,Henderson-Hasselbalch equation, acid-base titration, equivalence point, end point, indicator, titration curve Describe and explain the shape of the three types of titration curves as well as explaining the pH of the equivalence point Calculate the pH after the addition of a given amount of acid or base in a titration Calculate the pH at the equivalence point for a titration NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS A TITRATION? WHAT IS EQUIVALENCE POINT? WHAT IS AN END POINT? Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Section 3- 55,56,57,59,61,63,65,68,118,119,121,126 Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15PartI Learning Objectives: Students will be able to: Define common ion effect, buffer (or buffered solution) ,Henderson-Hasselbalch equation, acid-base titration, equivalence point, end point, indicator, titration curve Describe and explain the shape of the three types of titration curves as well as explaining the pH of the equivalence point Calculate the pH after the addition of a given amount of acid or base in a titration Calculate the pH at the equivalence point for a titration NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS A TITRATION? WHAT IS EQUIVALENCE POINT? WHAT IS AN END POINT? Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Section 3- 55,56,57,59,61,63,65,68,118,119,121,126 Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15PartI Learning Objectives: Students will be able to: Define common ion effect, buffer (or buffered solution) ,Henderson-Hasselbalch equation, acid-base titration, equivalence point, end point, indicator, titration curve Describe and explain the shape of the three types of titration curves as well as explaining the pH of the equivalence point Calculate the pH after the addition of a given amount of acid or base in a titration Calculate the pH at the equivalence point for a titration NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS A TITRATION? WHAT IS EQUIVALENCE POINT? WHAT IS AN END POINT? Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Section 4 – 69,70,71,73,75,77,122,132 Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15PartI Learning Objectives: Students will be able to: Define common ion effect, buffer (or buffered solution) ,Henderson-Hasselbalch equation, acid-base titration, equivalence point, end point, indicator, titration curve Describe and explain the shape of the three types of titration curves as well as explaining the pH of the equivalence point Calculate the pH after the addition of a given amount of acid or base in a titration Calculate the pH at the equivalence point for a titration NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT IS A TITRATION? WHAT IS EQUIVALENCE POINT? WHAT IS AN END POINT? Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Review for the test Free response questions TEST CHAPTER ACID BASE EQUILIBRIA Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15 PartII Learning Objectives: Students will be able to: solubility product constant, solubility, molar solubility, common ion effect, ion product, formation constant – complexes, dissociation constant – complexes Write solubility product constant (Ksp) expressions Determine the Ksp from the solubility of a substance Determine the solubility of a substance from its Ksp Perform solubility calculations involving the common ion effect Write ion product (Qc) expressions Use ion products to predict precipitation Recognize when the solubility of a substance will be affected by the pH Given a set of substances, state which substances will be more soluble in acidic solutions than in water Perform equilibrium calculations for the formation of a complex Calculate the solubility of a slightly soluble salt in a solution of the complex ion NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Section 1- 79,81,83,85,87,89,93,95, 97,98,99,100,101,123,126, Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15 Learning Objectives: Students will be able to: solubility product constant, solubility, molar solubility, common ion effect, ion product, formation constant – complexes, dissociation constant – complexes Write solubility product constant (Ksp) expressions Determine the Ksp from the solubility of a substance Determine the solubility of a substance from its Ksp Perform solubility calculations involving the common ion effect Write ion product (Qc) expressions Use ion products to predict precipitation Recognize when the solubility of a substance will be affected by the pH Given a set of substances, state which substances will be more soluble in acidic solutions than in water Perform equilibrium calculations for the formation of a complex Calculate the solubility of a slightly soluble salt in a solution of the complex ion NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework 103,104,105,106,107,108,109,110,111,112 Riar: A.P Chemistry UNIT 11: Acids & Bases Equilibria Chapter 15 part II Learning Objectives: Students will be able to: solubility product constant, solubility, molar solubility, common ion effect, ion product, formation constant – complexes, dissociation constant – complexes Write solubility product constant (Ksp) expressions Determine the Ksp from the solubility of a substance Determine the solubility of a substance from its Ksp Perform solubility calculations involving the common ion effect Write ion product (Qc) expressions Use ion products to predict precipitation Recognize when the solubility of a substance will be affected by the pH Given a set of substances, state which substances will be more soluble in acidic solutions than in water Perform equilibrium calculations for the formation of a complex Calculate the solubility of a slightly soluble salt in a solution of the complex ion NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – Development: Acids And Bases Chapter 15 Power point Summary/Assessment: Homework Take home test due Riar: A.P Chemistry UNIT 11: Thermodynamics (Spontaneity & Entropy) Chapter 16 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: First Law of Thermodynamics, spontaneous processes, nonspontaneous processes, entropy, Second Law of Thermodynamics, microstate, Third Law of Thermodynamics, absolute entropy, standard entropy, standard state, standard entropy of reaction, Gibbs free energy, standard free energy change of a reaction standard free energy of formation, thermodynamic equilibrium constant Give a molecular interpretation of entropy Relate entropy and disorder Relate entropy and temperature Apply qualitative rules for comparing entropy Use heat capacity to calculate entropy Predict the sign of the standard entropy of a reaction Calculate the standard entropy of reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE SPONTANEOUS PROCESSES ? WHAT IS ENTROPY? Development: Thermodynamics Chapter 16Power point Summary/Assessment: Homework Section 1 – Spontaneity and Entropy 17,18,21,23,25,27,28,29,31,32,33,34,35,36,37,67 Riar: A.P Chemistry UNIT 11: Thermodynamics (Spontaneity & Entropy) Chapter 16 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: First Law of Thermodynamics, spontaneous processes, nonspontaneous processes, entropy, Second Law of Thermodynamics, microstate, Third Law of Thermodynamics, absolute entropy, standard entropy, standard state, standard entropy of reaction, Gibbs free energy, standard free energy change of a reaction standard free energy of formation, thermodynamic equilibrium constant Give a molecular interpretation of entropy Relate entropy and disorder Relate entropy and temperature Apply qualitative rules for comparing entropy Use heat capacity to calculate entropy Predict the sign of the standard entropy of a reaction Calculate the standard entropy of reaction NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE SPONTANEOUS PROCESSES ? WHAT IS ENTROPY? Development: Thermodynamics Chapter 16Power point Summary/Assessment: Homework Section 2 – Calculations ΔS, ΔH and ΔG Riar: A.P Chemistry UNIT 11: Thermodynamics (Spontaneity & Entropy) Chapter 16 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: First Law of Thermodynamics, spontaneous processes, nonspontaneous processes, entropy, Second Law of Thermodynamics, microstate, Third Law of Thermodynamics, absolute entropy, standard entropy, standard state, standard entropy of reaction, Gibbs free energy, standard free energy change of a reaction standard free energy of formation, thermodynamic equilibrium constant Describe the effect of temperature on free energy Compare the enthalpy term and the entropy term in the Gibbs free energy equation at various temperatures Apply the rules of thumb regarding the signs of H, S, G and spontaneity calculate free energy changes from standard enthalpy and standard entropy calculate standard free energy from standard free energies of formation Calculate K from G NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE SPONTANEOUS PROCESSES? WHAT IS ENTROPY? Development: Thermodynamics Chapter 16 Power point Summary/Assessment: Homework Section 3 – Equilibrium 55,57,58,59,60,61,63,65,66,72,73,74,75 Riar: A.P Chemistry UNIT 11: Thermodynamics (Spontaneity & Entropy) Chapter 16 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: First Law of Thermodynamics, spontaneous processes, nonspontaneous processes, entropy, Second Law of Thermodynamics, microstate, Third Law of Thermodynamics, absolute entropy, standard entropy, standard state, standard entropy of reaction, Gibbs free energy, standard free energy change of a reaction standard free energy of formation, thermodynamic equilibrium constant Describe the effect of temperature on free energy Compare the enthalpy term and the entropy term in the Gibbs free energy equation at various temperatures Apply the rules of thumb regarding the signs of H, S, G and spontaneity calculate free energy changes from standard enthalpy and standard entropy calculate standard free energy from standard free energies of formation Calculate K from G NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE SPONTANEOUS PROCESSES? WHAT IS ENTROPY? Development: Thermodynamics Chapter 16 Power point Summary/Assessment: Homework Section 3- Equilibrium 76,77, 78, 79, 81 ,83, 84, 85 Riar: A.P Chemistry UNIT 11: Thermodynamics (Spontaneity & Entropy) Chapter 16 Learning Objectives: Students will be able to: Describe the effect of temperature on free energy Compare the enthalpy term and the entropy term in the Gibbs free energy equation at various temperatures Apply the rules of thumb regarding the signs of H, S, G and spontaneity calculate free energy changes from standard enthalpy and standard entropy calculate standard free energy from standard free energies of formation Calculate K from G NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – WHAT ARE SPONTANEOUS PROCESSES? WHAT IS ENTROPY? Development: Thermodynamics Chapter 16 Power point Summary/Assessment: Homework A.P Free response Questions – Take home Riar: A.P Chemistry UNIT 12: Electrochemistry Chapter 17 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: redox reaction, oxidation number, half-reaction, oxidation, reduction, electrochemical cell ,voltaic cell, also called a galvanic cell ,electrolytic cell ,half-cell ,salt bridge ,anode ,cathode cell reaction ,potential difference ,electromotive force ,standard electrode potential (E) ,standard hydrogen electrode (SHE) ,reduction potential ,oxidation potential ,standard reduction potential Left-Right-Below Diagonal Rule ,standard cell emf ’s (Ecell) ,volt ,coulomb ,joule ,Faraday’s constant ,free-energy-Faraday equation ,Nernst equation ,reaction quotient ,electrolysis, electrolytic cell ,electroplating ,redox titration Describe the construction of voltaic cells Compare and contrast anodes and cathodes Draw and label a voltaic cell Use cell notation for voltaic cells Determine the strengths of oxidizing and reducing agents Put a list of species in order of increasing strength as an oxidizing agent or reducing agent Predict the direction of spontaneity from standard electrode potentials Write reactions in the direction of spontaneity using the “Left-Right-Below Diagonal Rule” NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – HOW CAN CHEMICAL ENERGY BE CONVERTED TO ELECTRICAL ENERGY? WHAT IS AN GALVANIC CELL? Development: Thermodynamics Chapter 17 Power point Summary/Assessment: Homework Section 1 Galvanic Cells 25,33,35,47,49.51,52,53,99,100,101,107 Riar: A.P Chemistry UNIT 12: Electrochemistry Chapter 17 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: redox reaction, oxidation number, half-reaction, oxidation, reduction, electrochemical cell ,voltaic cell, also called a galvanic cell ,electrolytic cell ,half-cell ,salt bridge ,anode ,cathode cell reaction ,potential difference ,electromotive force ,standard electrode potential (E) ,standard hydrogen electrode (SHE) ,reduction potential ,oxidation potential ,standard reduction potential Left-Right-Below Diagonal Rule ,standard cell emf ’s (Ecell) ,volt ,coulomb ,joule ,Faraday’s constant ,free-energy-Faraday equation ,Nernst equation ,reaction quotient ,electrolysis, electrolytic cell ,electroplating ,redox titration Describe the construction of voltaic cells Compare and contrast anodes and cathodes Draw and label a voltaic cell Use cell notation for voltaic cells Use the Left-Right-Below Diagonal Rule to determine spontaneity for reactions already given Combine half-reactions to determine standard cell emf ’s (Ecell) Write cell reactions Calculate free-energy changes from emf ’s Calculate emf’s from free-energy changes NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – HOW CAN CHEMICAL ENERGY BE CONVERTED TO ELECTRICAL ENERGY? WHAT IS AN GALVANIC CELL? Development: Thermodynamics Chapter 17 Power point Summary/Assessment: Homework Section 2 & 3 Calculations cell potential, ΔG and Nernst Equation 39,41, 43, 403, 108, 109, 110, 113 55,59,61,63,67,68,71,72,75,77,114 Riar: A.P Chemistry UNIT 12: Electrochemistry Chapter 17 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: redox reaction, oxidation number, half-reaction, oxidation, reduction, electrochemical cell ,voltaic cell, also called a galvanic cell ,electrolytic cell ,half-cell ,salt bridge ,anode ,cathode cell reaction ,potential difference ,electromotive force ,standard electrode potential (E) ,standard hydrogen electrode (SHE) ,reduction potential ,oxidation potential ,standard reduction potential Left-Right-Below Diagonal Rule ,standard cell emf ’s (Ecell) ,volt ,coulomb ,joule ,Faraday’s constant ,free-energy-Faraday equation ,Nernst equation ,reaction quotient ,electrolysis, electrolytic cell ,electroplating ,redox titration Combine half-reactions to determine standard cell emf ’s (Ecell) Write cell reactions Calculate free-energy changes from emf ’s Calculate emf’s from free-energy changes Calculate the equilibrium constant from emf ’s Calculate Ecell under nonstandard conditions Describe the electrolysis of various substances Describe the electroplating of metals Calculate stoichiometry problems involving electrolysis Describe the similarities between acid-base titrations and redox titrations Discuss the key points to redox titrations NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – HOW CAN CHEMICAL ENERGY BE CONVERTED TO ELECTRICAL ENERGY? WHAT IS AN GALVANIC CELL? Development: Thermodynamics Chapter 17 Power point Summary/Assessment: Homework Section 4 Electrolysis 79,81,83,85,86,87,89,94,95,98,111,116 Riar: A.P Chemistry UNIT 12: Electrochemistry Chapter 17 Learning Objectives: Students will be able to Define, compare and contrast, classify, differentiate, put in order, predict using, identify correct and incorrect examples of, identify correct and incorrect interpretations of, identify correct and incorrect applications of…the following terms: redox reaction, oxidation number, half-reaction, oxidation, reduction, electrochemical cell ,voltaic cell, also called a galvanic cell ,electrolytic cell ,half-cell ,salt bridge ,anode ,cathode cell reaction ,potential difference ,electromotive force ,standard electrode potential (E) ,standard hydrogen electrode (SHE) ,reduction potential ,oxidation potential ,standard reduction potential Left-Right-Below Diagonal Rule ,standard cell emf ’s (Ecell) ,volt ,coulomb ,joule ,Faraday’s constant ,free-energy-Faraday equation ,Nernst equation ,reaction quotient ,electrolysis, electrolytic cell ,electroplating ,redox titration Combine half-reactions to determine standard cell emf ’s (Ecell) Write cell reactions Calculate free-energy changes from emf ’s Calculate emf’s from free-energy changes Calculate the equilibrium constant from emf ’s Calculate Ecell under nonstandard conditions Describe the electrolysis of various substances Describe the electroplating of metals Calculate stoichiometry problems involving electrolysis Describe the similarities between acid-base titrations and redox titrations Discuss the key points to redox titrations NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: ASK STUDENTS – HOW CAN CHEMICAL ENERGY BE CONVERTED TO ELECTRICAL ENERGY? WHAT IS AN GALVANIC CELL? Development: Thermodynamics Chapter 17 Power point Summary/Assessment: Homework A.P Free response Questions – Take home Review For the A.P Exam May 2 UNIT 13: Final AP CHEMISTRY PROJECT End of the year project: Guidelines Learning Objectives: The objective of this project is to allow students to explore aspects of Chemistry you haven’t yet experienced. Specifically, research based experimentation, lab design, data analysis, and (most importantly) science education. You and your partner will be assigned a Lab experiment to explore the problem, conduct your experiment, analyze your data, and finally teach the experiment to the entire class. You will be assessed in two ways: First, I will grade your lab’s procedure and analysis. Then, you will be graded by me and the rest of the class on the quality of your lesson. This one project represents the majority (50%) of your 4th quarter grade and ALL of you final exam grade. NJCCC standards addressed: 5.2.12.A.5 Describe the process by which solutes dissolve in solvents. 5.2.2.A.1 Sort and describe objects based on the materials of which they are made and their physical properties. 5.2.8.A.1 Explain that all matter is made of atoms, and give examples of common elements 5.2.8.A.2 Analyze and explain the implications of the statement “all substances are composed of elements.” 5.2.6.B.1 Compare the properties of reactants with the properties of the products when two or more substances are combined and reaction 5.2.8.B.1 Explain, using an understanding of the concept of chemical change, why the mass of reactants and the mass of products 5.2.12.B.3 Balance chemical equations by applying the law of conservation of mass. Motivation: Development: Each Group will : 1) Try out the experiment and modify it if necessary to get it working. This means that you must prepare all solutions and get all the equipment needed. You may have to learn additional theory and use other sources to get more information. THE LAB MUST WORK. Perform the experiment sufficient number of times so that you understand what you are doing and so that you have enough consistent data to allow your group to reach a conclusion. A single run generally means nothing. Summary/Assessment: 1) Prepare a written Lab write –up for distribution to the class. The write up should contain the experiment’s purpose an introduction, a procedure, a data table and a place to do the calculations. You also need some form of activity for the class to do. It could be asset of questions or an overall theory statement so that each individual in the rest of the class is responsible for doing something which shows that they have learned something. Make copies of the lab write up for the class. 2) Students will be prepared on the due date to teach the lesson in which all members of the group teach the lab’s principles and concepts and how the experiment was done. You may want to show the experimental set up and how the experiment was conducted and data was collected. You will need to take the class through calculations and the final result. This presentation will require at least a class period and may require more time. It must be rehearsed with Mrs. Riar at least two days before the Final presentation. 3) Each group will turn in complete set of teacher notes describing how to make all of the solutions, showing all of your experimental data and your calculations and graphs as well as your results Every group is the teacher for every aspect of their experiment. Both members of the group are expected to share all parts of this lab experiment equally. Homework Proposed Presentation dates Assigned Experiments: Group 1 Complex ion Ag(NH3)2 Group 2 Avogadro’s Group 3 Redox Group 4 Ksp of PbCl2 Presentation date June 1st Presentation date June 2nd Presentation date June 6th Presentation date June 7th May 7th onwards: Before students make any solutions they will do the calculations and show all the calculations Make solutions and do the experiment. Calculate results. Repeat the experiment to refine their technique and get improved results and additional data.