Chem 340 Fall 2013 – Lecture Notes 12
advertisement

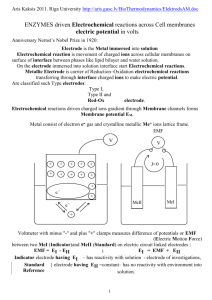
Chem 340 Fall 2013 – Lecture Notes 12- Electrochemistry (Chap. 6) Charged particle energies affected by applied electric fields, similarly dissolution of metals from electrodes to create ions also creates a potential difference between electrode and solution, e.g. Zn0 (s) Zn+2 (aq) + 2eSolution gets positive and electrode negative (e- left), but the electrical potential that develops is dependent on the metal and ion and affects chemical potential of charged species in solution dwrev = (2 –1) dQ, where dQ = - zF dn where F is a Faraday = 96485 C/mol (charge on mol e-) this is reversible, non-expansion work, so dG = dwrev 1 – 2z dn where z is # charge transfer zelectrochemical potential, 0 2 2z – 1z = 2z – 1z + z (2–1)F plug in def., drop dn but 2 = 1 i.e. same thing (metal), different phases (diff. ), so 2 – 1 = + z(2 – 1)F can only measure difference in potential, let 1=0 2 = 1 + z 2 F means charged particles differ in chemical potential by Charge will flow to reduce potential, negative particles toward more positive region Chemist can control chemical potential of charged particles, even change sign of Grxn Work: Electrochemical cells Example shown right, has two half-cells, each with different metal electrode and a salt solution (dissociated) with ions of the same metal. Electrodes connected with meter to measure electrical potential, s ts k w th “s t b ” – electrolyte (e.g. KCl) in a gel (not moving much). Note, substitute power supply c “p t out” m t s f om s t so ut o . Metal—salt equilibrium makes different potential each side, but need both to get measurement. Establish standard state, at = 0 for ions in solution or M+M+(as before), with field: M+M+ + zF for e- in electrode e= 0, eF, and M M At equil.: M M++ eM+ + zFzFM+ Add components, note - metal pure element so MM+=0 and M+M+ + zF = zF bottom line, chem pot. depend on electrical potential 1 Still only measure difference in cells, reference to: Standard Hydrogen Electrode, SHE H+(aq) + e- ½ H2(g), at equilibrium: H+ + e- = ½ H2 f~a For unit activities: since H2 = 0 and Gfo(H+) = 0, reference electrode Need standard concentration, aH+ = 1 Pt electrode catalyze H2 2H+ +2e- SHE Measure cell potential against SHE and get Zn/Zn+2 potential for the half-cell reaction, in figure potential is for the Zn/Zn+2 half-cell, collect relative potentials to SHE and then can determine cell potentials relative to each other shorthand: (-) Zn|Zn+2||H+|H2|Pt (+) cell previous page Left : Zn(s) Zn+2(aq) + 2eoxidation anode +2 Right: Cu (aq) +2e Cu(s) reduction cathode +2 +2 e balance out Sum: Zn(s) + Cu (aq) Zn (aq) + Cu(s) or: Zn|Zn+2||Cu+2|Cu chem. potentials: Grxn = Zn2+ Cu - Cu2+ – Zn = Zn2+ - Cu2+ + RTln(aZn2+/aCu2+) ionGion~ Grxn = Gorxn + RTln(aZn2+/aCu2+) = -nF Grxn ~ emf o – emf - electromotive force: G rxn = -nF generalize RT/nF )RTln(aM1/aM2) Nerst equation: RT/nF) RTln(Q) or 0.05916/n) log10(Q) T=298K If know activities and o then can calculate cell potential Current? 1 mole of Zn0 Zn+2, then 2 mole e- flow, 1 mole Cu+2 Cu0 (not normal use) Also works for half-cells, Ox +ne- Red: Combining half-cells: Tables list reduction potentials, but ored = -oox So cell potential is: ocell =ored + oox since Grxn = -nFthen Grxn = -nF Clearly, if process, reaction spontaneous, then o > 0 2 Examples Engel 9.4,9.5 Eo’ = -0.320V Eo’ = -0.414V Eo’ = 0.295V Erxn’o = - 0.320 + (+ 0.414V) = 0.096 V (same since 1H+ in both sides) also Eoxo’= - Eredo’ Change coupled half-cell can reverse the NAD + H+ +e- NADH reaction, flip order Erxn’o = - 0.320 + (- 0.295V) = -0.615 V Red. pot. for NAD+, so Eoxo’ = +0.615V (sign same value differ, 2H+ for H2O) Entropy determine from temperature variation of o: Enthalpy can be obtained from G = H – TS H = G + TS Combine K and measurement: RT/nF) RTln(Q) =RT/nF) RTln(K/Q) 3 Engel Example 9.5 S (-) could be expected for gas to solvated ion At equilibrium, = 0, rewrite Nernst: o = (RT/nF) ln K standard potential gives K, but in principle need unit activities to determine o, alternate: combine half-cell values + - Pt|NAD |NADH||HCOO |CO2|Pt NAD+ +HCOO- CO2 + NADH NAD+ +H+ +2e- NADH o = -0.105 V HCOO- CO2 + H+ +2eo = 0.200 V Grxn = -nF = - 2(96485 C/mol)(-0.105 + 0.200)V = -18.3 kJ/mol ln K = -G/RT = 18.3 kJ/mol/(8.314 x 298 J/mol) = 7.4 K = exp(7.4) = 1640 reaction strongly favors products, challenging to measure equil. conc. using ordinary methods (eg spectroscopy) but with electrochem, trivial to get value with voltmeter Engel Example 9.6 – more dramatic In this case, no reactants left, but potential still easy to measure 4 Solubility products (view salt dissolution as two steps): AgBr(s) + e- Ag(s) + Br -(aq) Eo = 0.07133 Ag(s) Ag+(aq) + eEo = -0.7996 AgBr(s) Ag+(aq) + Br-(aq) Eo = -0.7283 ln Ksp = (nF/RT )Eo = (1x96485 C/mol)(-0.7283 V)/(8.314J/molK)(298 K) = -28.4 Ksp = 4.9x10-13 here not measure conc. directly, except electrode and voltmeter easy Determine activity coefficients, since Debye Hückel works at low concentrations and since ± 0 at low concentrations then measuring at low concentrations will have less error from activity coefficients. = o + RT/F ln a± = o + RT/F ln b± +RT/F ln ± As decrease concentration, Debye Hückel for 1:1, I = b±: ln ± = -1.173 (b±)½ at T=298K: – 0.02568 ln b± = o – 0.03013 (b±)½ or – 0.05916 log10b± = o – 0.03013 (b±)½ or (m±)½ Plot: ( – 0.05916 log10b±) vs (b±)½ and extrapolate b0 This gives o – use this and concentration to calcuate ± - most accurate values Biochemical standard state, since aoH+ = 10-7 standard instead of aoH+ = 1 will impact the equilibrium constant, e.g. rR pP + vH+ then normally: K = (aP/ao)p(aH+/aoH+)v/(aR/ao)r If chemical standard state all the ao values are 1, K = (aP)p(aH+)v/(aR)r but if use biochem standard state, K’ ( P)p(aH+/10-7)v/(aR)r so K’/K 107v Relate to Go: Go’ -RT K’ - RTln K – 7vRT ln10 = Go – 7vRT ln10 Electrochemical cell same: o’ -Go’/ F (RT/ F) K’ (RT/ F)( K 7vRT 10) o’ = o + 7v(ln10)(RT/nF) tables in chemical reduction potentials or biochemical reduction potentials (below) ’(V) o Reaction 5 Thermodynamic cycles and emf, cell potentials are intrinsic quantities (only depend on concentration not how much you have), but G is extrinsic (Go’ f s p mo ) o o G ’ - nF ’ So if combining reactions in a thermodynamic cycle, need to account for quantity (n) 6 CH3COO- + 3H+ + 2e- CH3CHO + H2O Eo’ -0.581 V CH3CHO + 2H+ + 2e- CH3CH2OH Eo’ -0.197 V Calculate Eo’ fo combined half-cell: CH3COO- + 5H+ + 4e- CH3CH2OH + H2O Example: “Easy way” sum the Eo’ v u s but ov cou ts since overall rxn is 4e-, parts are 2eIf convert to Go’ F o’ fo ch th sumGo’ s usu b ck co v t to o’ G1o’ = -nF 1o’ -2x96485C/mol(-0.581V) = 112 kJ/mol G2o’ -nF 2o’ -2x96485C/mol(-0.197V) = 38 kJ/mol Go’ G1o’ G2o’ (112 38) kJ/mo 150kJ/mo o’ Go’ / F - 0.388V Note: this is actually (1o’ 2o’ )/2 (-0.581-0197)V/2 = -0.389 V General: for A+nAe- B+nBe- C: (nA+nA)A/Co’ nAA/Bo’ nBB/Co’ Transmembrane effects Cellular membranes (lipid bilayer) are relatively impervious to ions and transport of charged species and can maintain a charge/potential difference Transport of ions usually incorporates channels and proteins that act as pumps Work in elect. transport: wE = Q, and GE = ZF, for moving ion of charge z Difference in chemical potential then : =E + C = zF + RT lnQ At equilibrium, chem. pot. difference : = 0 or zF = - RT lnK or = - (RT/zF) lnK Example: mammalian plasma membrane has = in - out ~ -70mV. It has pores to allow K+ to equilibrate. If extracellular [K+] = 5mM, what is concentration inside the cells at 37oC, assume unit activity coefficients For K+, z=1, RT/zF = 8.314x310/96485 = 0.0267 V So lnQ = - /(RT/zF) = - (-0.07)/0.0267 = 2.62 and Q = 13.7 or [K+in] =13.7 [K+out] = 13.7x0.005 M = 69 mM Donnan effect: separate two solutions with a semipermeable membrane that will let small ions, e.g. Na+ and Cl-, pass but will block macromolecules, e.g. Protein dialysis put salt solution on one side, protein solution on other, and assume protein is z:1 salt, i.e. dissociate to P-z + zNa+, and start with: bNazP on side 1, aNaCl side 2, at equilibrium, get; (zb+x)Na+, xCl- and bP-z on side 1 and (a-x)Na+, (a-x)Cl- on side 2 also at equilibrium 1± = 2± and std. state also: 1± = 2± so a1± = a2± or a1+a1- = a2+a2- if ± = 1, i.e. very dilute: c1+/c2+ = c1-/c2- =1 would work if no protein, since Na+ and Cl- would go through membrane and equalize Donnan effect: equilibrium c1+/c2+ = c1-/c2- = rD ≠ 1 where rD is Donnan ratio At equilibrium have (assume ± = 1): c1+c1- = c2+c2- or (zb+x)x = (a-x)(a-x) The Na+ and Cl- both go through membrane to maintain charge neutrality, but protein concentration is a constant (b), solve to get: x = a2/(2a+zb) and get all conc. 7 rD = c1-/c2- = [a - a2/(2a+zb)]/[a2/(2a+zb)]= [(a(2a+zb)-a2)/(2a+zb)]/[a2/(2a+zb)] = = [(a2+azb)/a2] = [(a+zb)/a] = rD show it’s same for c1+/c2+ = rD Put in electrodes, cell at equilibrium so = 0, but can still be described by Nernst = D+(RT/F)ln(c1+/c2+) = D+(RT/F)ln rD = 0 D = - (RT/F)ln rD =D + (RT/F)ln[(a+zb)/a] where D is the Donnan potential Physically this arises due to the difference in concentration of a given ion on either side and manifests itself as a polarization in the membrane Since concentrations differ on both sides of membrane, an osmotic pressure develops: net = (c2-c1)RT = [(1+z)b + 2x -2(a-x)]RT = [(1+z)b-2a+4x]RT = = [((1+z)b-2a)+4a2/(2a+zb)]RT = [(zb2+2ab+z2b2)/(zb+2a)]RT =net Cells are permeable to small ions and not to proteins or nucleic acids in general. Concentration of anionic proteins above described is passive, can be high in cell, would cause a rupture except that sodium/potassium pump (in membrane) changes concentration outside/inside to counter net – active transport balance it out Problem need to transport against chem pot, so need to do work or consume energy ATPase Na-K pump helps this: 3Nain+ +2Kout+ +ATP 3Naout+ +2Kin+ +ADP + Pi Can be inhibited by cardiotonic steroids, e.g. digitoxin or other poisons, initially stimulate heartbeat then can shut it down 8 Practical note – this is a high single protein concentration (100 mM), but not so high for multiprotein containing cell Batteries – opposite: consume chemical reagents to get energy (electrical work) Wet cell, storage battery, 4 e- oxidation between Pb and PbO2, use PbSO4 as medium Pb + HSO4- PbSO4 + H+ + 2eox PbPb+2 o = -0.126 2e- + PbO2 + 3H+ + HSO4- PbSO4 + 2H2O red Pb+4 Pb+2 o = 1.45 Pb + PbO2 + 2H+ + 2HSO4- 2PbSO4 + 2H2O o = -0.126 -1.45 = -1.57V Actual V depend on concentration and temperature, Nernst: RT/nF) RTln(Q) Use PbSO4 to maintain high Pb+2 conc~const, and large excess H2SO4 Zn + xNH3 [Zn(NH3)x]+2 + 2e2MnO2 + 2NH4+ +2e- Mn2O3 + H2O + 2NH3 Done with salts in paste (conduct) with Zn as outer can, C-electrode in center, ~ 1.6 V Dry cell: 9