3. Diffusion of solutes in a porous solid Solute molecules can diffuse
advertisement

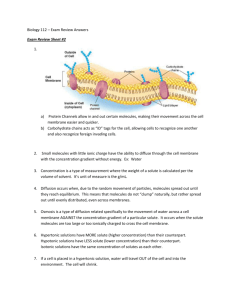
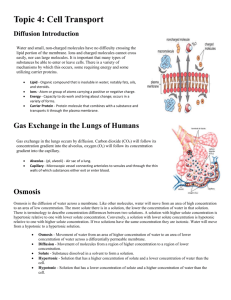
3. Diffusion of solutes in a porous solid Solute molecules can diffuse through the pores present in porous solids. In order for this to happen, the pores have to be filled with some liquid medium. Therefore no diffusion takes place through the solid material itself. All it does is hold the liquid medium in place. However, the solid material can have an influence on the diffusion within the liquid medium. It can for instance increase the effective diffusion path length of the solute if the pores are tortuous in nature. When the pores have dimension of the same order of magnitude as the solute, the pore wall can cause hindrance to diffusion. An example of un-hindered diffusion in a porous medium is the transport of sodium chloride through a microfiltration membrane (which has micron sized pores) while an example of hindered diffusion is the transport of albumin through an ultrafiltration membrane (which has nanometer sized pores). The steady state equation for unhindered diffusion of a solute from point 1 to 2 within a slab of porous solid is given by: Where The hindered diffusion of a solute through a porous solid from point 1 to 2 is given by: Where Deff = effective hindered diffusivity (m2/s). The effective hindered diffusivity of a solute in a pore can be obtained by: Where ds = solute diameter (m) dp = pore diameter (m) It should be appreciated that when the pore size is very large as compared to particle or molecular size, the term in the parenthesis will essentially equal 1, and thus: Deff = D Which is of course the case for unhindered diffusion. Example Glucose is diffusing at 25 degrees celsius in water within a porous medium having a porosity of 0.5, tortuosity of 1.8 and average pore diameter of 8.6x 10-3 microns. Determine the steady state flux of glucose between two points within the medium separated by a distance of 1 mm and having concentrations 1.5 g/L and 1.51 g/L respectively. Solution Table 2.2, gives the molecular weight of glucose as being 180 kg/kgmole. The diffusivity of glucose at 25 degrees centigrade can be determined using Poison correlation: The size of glucose can be obtained from appropriate tables. The effective diffusivity of glucose in the porous structure can be obtained using the equation: The steady state flux of glucose can be obtained using equation: The molar concentration of glucose = (0.01/180) mol/L Convective mass transfer Convective transport occurs when a constituent of the fluid is carried along with the fluid. The amount carried past a plane of unit area perpendicular to the velocity (the flux) is the product of the velocity and the concentration NA = kcA(C0 – CA), or NA = kcA∆CA Where NA is the flux in convective mass transfer kcA is the convective mass transfer coefficient (C0 – CA) is the solute concentration difference between two points Convective mass transfer is observed in flowing fluids can be emphasized for two situations: 1. Transport of a solute in a liquid flowing past a solid surface (see Fig. 3.5) 2. transport of a solute in a liquid flowing past another immiscible liquid (see Fig. 3.6). An example of the first type is the transfer of urea from blood towards the surface of a dialyser membrane in haemodialysis. An example of the second type is the transfer of penicillin G within filtered aqueous media flowing past an organic solvent in a liquid-liquid extractor. When a liquid flows past a solid surface a stagnant boundary liquid layer is formed close to the surface. Similarly when two liquids flow past one another, two boundary liquid layers are generated on both sides of the interface. Within these boundary layers, the transport of solute mainly takes place by molecular diffusion. If the flow of liquid is laminar, the transfer of solute in the directions indicated in Figs. 3.5 and 3.6 would be by molecular diffusion. However, if the flow were turbulent in nature, mass transfer would take place by a combination of molecular diffusion and eddy diffusion. This is referred to as convective mass transfer. Another argument of convective mass transfer can be mentioned for a solute band progresses along a column, where the solute molecules are continually transferring from the mobile phase into the stationary phase and back from the stationary phase into the mobile phase. This transfer process is not instantaneous, because a finite time is required for the molecules to traverse (by diffusion) through the mobile phase in order to reach, and enter the stationary phase. Thus, those molecules close to the stationary phase will enter it almost immediately, whereas those molecules some distance away from the stationary phase will find their way to it a significant interval of time later. However, as the mobile phase is moving, during this time interval while they are diffusing towards the stationary phase boundary, they will be swept along the column and thus dispersed away from those molecules that were close and entered it rapidly. The dispersion resulting from the resistance to mass transfer in the mobile phase is depicted in the figure below. The diagram shows 6 solute molecules in the mobile phase and those closest to the surface (1 and 2) enter the stationary phase immediately. During the period, while molecules 3 and 4 diffuse through the mobile phase to the interface, the mobile phase moves on. Thus, when molecules 3 and 4 reach the interface they will enter the stationary phase some distance ahead of the first two. Finally, while molecules 5 and 6 diffuse to the interface the mobile phase moves even further down the column until molecules 5 and 6 enter the stationary phase further ahead of molecules 3 and 4. Thus, the 6 molecules, originally relatively close together, are now spread out in the stationary phase. This explanation is a little oversimplified, but gives a correct description of the mechanism of mass transfer dispersion. The same phenomenon occurs when solutes transfer between two immiscible moving liquids in contact with one another.