
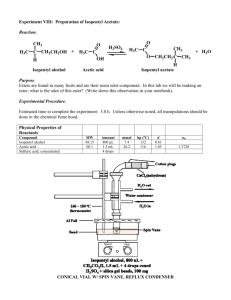
This page is intentionally left blank. OOC_CH02_049-058.pdf 1 8/19/2013 11:15:17 AM 50 Operational Organic Chemistry EXPERIMENT Preparation of Synthetic Banana Oil Simple Distillation, Gas Chromatography 2 Preparation, Purification, and Analysis of Liquids. Gas Chromatography. Operations OP-10 OP-24 OP-25 OP-30a OP-37 OP-2 OP-4 OP-7 Mixing (optional) Washing Liquids Drying Liquids Distillation of Liquids Gas Chromatography Using Specialized Glassware Weighing Heating Before You Begin 1. Read the experiment and operations OP-10 (if you’ll be using a magnetic stirrer), OP-24, OP-25, OP-30a, and OP-37. Read or review the other operations as necessary. (Reading OP-30a and OP-37 may be deferred until the second lab period for this experiment.) 2. Calculate the mass and volume of 150 mmol of isopentyl alcohol and the theoretical yield of isopentyl acetate from this amount of the alcohol. 3. Read Appendix V, “Planning an Experiment,” and then write an experimental plan like the one you used in Experiment 1. Scenario Your supervisor has just received the following message from the Cavendish Distilling Company. banana tree OOC_CH02_049-058.pdf 2 Greetings: We have a problem. Cavendish Distilling Company markets the popular liqueur Banana Elixir, which is flavored with a natural banana extract from fruit grown on our Caribbean banana plantations. Last June, Hurricane Floyd blew down all of our banana trees. Our stock of banana extract is running low, and we have no alternative source of bananas at this time.As a temporary solution, we have decided to add a synthetic banana flavoring to our remaining stock of the natural extract until our plantations start producing again. Synthetic banana flavorings are formulated mainly from isopentyl acetate, with smaller amounts of other esters. I understand that esters can be prepared economically by a process called the Fischer esterification, which involves the combination of an acid, 8/19/2013 11:15:17 AM Experiment 2 51 Preparation of Synthetic Banana Oil such as acetic acid, with an alcohol. According to our technical staff, one problem with this method is that Fischer esterifications don’t go to completion, leaving considerable amounts of starting material in the product. We can tolerate up to 10% isopentyl alcohol in our isopentyl acetate, since the alcohol is a component of natural banana extract. But we cannot tolerate more than 2% acetic acid, because it would tend to degrade the flavor of the liqueur. Will you have your consulting chemists prepare some isopentyl acetate and analyze it to see if it falls within our tolerances? Amy Lester, CEO The banana plant (Musa cavendishii and other species) is actually a gigantic herb and not a true tree. Each year, the foliage-bearing part withers away and is replaced by new growth from an underground stem. Applying Scientific Methodology By now, you should be familiar with the steps involved in applying scientific methodology to the solution of a problem, so they won’t all be repeated in subsequent experiments. In this experiment, the problem described in the Scenario involves the purity of the product—not its identity—so your course of action will include the use of an instrumental method, gas chromatography, to determine the composition of the product. Esters and Artificial Flavorings The word flavor is used to describe the overall sensory effect of a substance taken into the mouth. Flavor may involve tactile, temperature, and pain sensations, as well as smell and taste. Many fruits, flowers, and spices contain esters that contribute to their characteristic flavors—an ester is an organic compound that contains the functional group (characteristic combination of atoms) shown in the margin. Most volatile esters have strong, pleasant odors that can best be described as “fruity.” Some esters with flavors characteristic of real and “fantasy” fruits are shown in Table 2.1. The ester you will prepare in this experiment, isopentyl acetate, has a strong banana odor when undiluted and an odor reminiscent of pears in dilute solution. It is used as an ingredient in artificial coffee, butterscotch, and honey flavorings, as well as in pear and banana flavorings. Many different esters are included in the basic repertoire of the flavor chemist, who combines natural and synthetic ingredients to prepare artificial flavorings. These ingredients may include natural products, synthetic organic compounds identical to those found in nature, and synthetic compounds not found in nature but accepted as safe for use in food. Each flavor ingredient is characterized by one or more flavor notes that suggest the predominant impact the ingredient makes on the senses of taste and smell. Although the flavor note of a single ingredient may seem unrelated to the overall character of a natural flavor, the combination of carefully selected ingredients in the right proportions can often yield a good approximation of that flavor. A high-boiling fixative, such as glycerine or benzyl benzoate, is usually added to an artificial flavoring to retard vaporization of volatile components, and the flavor notes of the individual components are blended by dissolving them in a solvent called the vehicle. The most frequently used vehicle is ethanol (ethyl alcohol). OOC_CH02_049-058.pdf 3 O C O R functional group of an ester O CH3COCH2CH2CHCH3 CH3 isopentyl acetate Some food products, such as cola beverages and Juicy Fruit gum, are characterized by fantasy flavors that have no counterparts in nature, but most artificial flavorings are meant to resemble natural flavors. 8/19/2013 11:15:17 AM Operational Organic Chemistry 52 Table 2.1 Flavor notes of some esters used in artificial flavorings Name Structure Flavor note O propyl acetate CH3C OCH2CH2CH3 pears O(CH2)7CH3 oranges OCH2 peaches, strawberries O octyl acetate CH3C O benzyl acetate isopentenyl acetate isobutyl propionate CH3C O CH3C CH3 OCH2CH O CH3CH2C C CH3 “Juicy Fruit” CH3 rum CH3 OCH2CH O ethyl butyrate Fixatives OH OHOH CH2CHCH2 glycerine O COCH2 benzyl benzoate Vehicle CH3CH2OH ethyl alcohol CH3CH2CH2C OCH2CH3 pineapples The formulation of artificial flavorings is perhaps as much an art as a science. The components of a strawberry flavoring, for example, may vary widely depending on the manufacturer and the specific application. Because natural flavors are usually very complex, a cheap artificial flavoring may be a poor imitation of its natural counterpart, but advances in flavor chemistry have made possible the production of superior flavorings that reproduce natural flavors very closely. Superior flavorings may contain natural oils or extracts that have been fortified with a few synthetic ingredients to enhance the overall effect and to replace flavor elements lost during the distillation or extraction process. Even the most experienced flavor chemist can’t hope to do as well as a strawberry plant, which may combine several hundred different flavor components in its berries. But a superior strawberry flavoring with a few dozen ingredients may be hard to distinguish from the real thing—except by the most discriminating of strawberry aficionados. Understanding the Experiment pear In this experiment you will prepare synthetic banana oil, which is known by several chemical names, including isopentyl acetate, isoamyl acetate, isopropyl acetate and 3-methylbutyl ethanoate. Esters are often prepared by the Fischer esterification method, which involves heating a carboxylic acid with an alcohol in the presence of an acid catalyst, as shown by the following OOC_CH02_049-058.pdf 4 8/19/2013 11:15:17 AM Experiment 2 53 Preparation of Synthetic Banana Oil general equation: O RCOH carboxylic acid O + HOR⬘ alcohol + H RCOR⬘ + H2O ester The acid catalyst is used to increase the rate of the reaction, which would otherwise require a much longer reaction time. isopropyl acetate You will synthesize isopentyl acetate by combining isopentyl alcohol (3-methyl-1-butanol) with acetic acid and sulfuric acid and then heating the reaction mixture under reflux for an hour.The alcohol is the limiting reactant, so it should be weighed; the acids can be measured by volume. The esterification reaction is reversible, and it has an equilibrium constant of approximately 4.2. If you were to start with equimolar amounts of acetic acid and isopentyl alcohol, only about two-thirds of each reactant would be converted to isopentyl acetate by the time equilibrium was reached.Your highest attainable yield in that case would be only 67% of the theoretical value. Thus, despite having a high atom economy, the reaction efficiency of the synthesis could be rather low. To increase the reaction efficiency, you will apply Le Châtelier’s principle by using a 100% excess of acetic acid—the less expensive reactant—to shift the equilibrium toward the products. Even then, the reaction will not be complete at equilibrium, so the reaction mixture will contain some unreacted isopentyl alcohol as well as the excess acetic acid. A desired substance can be obtained from a mixture by separating it from all other components of the mixture, using procedures that take advantage of differences in solubility, boiling points, acid–base properties, and other characteristics of the components. Because isopentyl acetate is a liquid, the separation and purification operations will differ from those used previously for solid products. At the end of the reflux period, the reaction mixture will contain (in addition to the ester) unreacted acetic acid, sulfuric acid, water, unreacted isopentyl alcohol, and some unwanted by-products (see Figure 2.1). Isopentyl acetate is quite insoluble in water, whereas both acetic acid and sulfuric acid are water soluble and acidic. This makes it easy to separate the two acids from the product by washing the reaction mixture with water and then with aqueous sodium bicarbonate.Water doesn’t remove the acids entirely, because they are somewhat soluble in the ester as well, but it removes the bulk of them and thus helps prevent a violent reaction with sodium bicarbonate in the second washing step. The aqueous sodium bicarbonate converts the acids to their salts, sodium acetate and sodium sulfate, which are insoluble in the ester but very soluble in water; these salts migrate to the aqueous layer, where they can be removed. The rule of thumb given in OP-24 will help you estimate the quantity of aqueous sodium bicarbonate needed. The water that forms during the reaction will be separated from the ester along with the wash liquids. Any traces of water that remain are then removed by a drying agent, either magnesium sulfate or sodium sulfate. The rule of thumb given in OP-25 will help you estimate the amount of drying agent needed. OOC_CH02_049-058.pdf 5 H2SO4 as acid catalyst high atom economy, but low efficiency Key Concept: For a reaction at equilibrium, adding more of a reactant or removing a product will shift the equilibrium to favor the products. aqueous: H2SO4, water, acetic acid, 2-propanol organic: isopropyl acetate Reactions carried out with acid catalysts often yield polymeric, tarlike byproducts that have high boiling points and are insoluble in water. 8/19/2013 11:15:17 AM 54 Operational Organic Chemistry acetic acid isopentyl alcohol sulfuric acid REACTION MIXTURE Reflux PRODUCT MIXTURE isopentyl acetate acetic acid* isopentyl alcohol* sulfuric acid water by-products Wash aqueous layers acetic acid sulfuric acid water as salts organic layer isopentyl acetate isopentyl alcohol water (trace) by-products solid residue water—as hydrate Dry isopentyl acetate isopentyl alcohol by-products forerun, residue Distill isopentyl acetate isopentyl alcohol by-products *unreacted starting materials Figure 2.1 Flow diagram for the synthesis of isopentyl acetate Because isopentyl alcohol has a lower boiling point than that of isopentyl acetate, and the by-products have higher boiling points, it should be possible—in principle—to remove the alcohol and by-products from the ester by distillation. Isopentyl alcohol should distill first, followed by the ester, and any by-products should remain behind in the pot—the vessel in which the reaction mixture is boiled. For the reasons described in OP-30a, the separation is incomplete, so you will still have some isopentyl alcohol in your isopentyl acetate after the purification step. You will determine the composition of your distillate by injecting a very small amount into an instrument called a gas chromatograph. (Your instructor will demonstrate the operation of this instrument.) Inside the gas chromatograph, the liquid will vaporize, and the vapors of different components will travel through a packed column at different rates. As the vapors exit the column, their presence will be detected and recorded on a graph called a gas chromatogram, which should display several peaks of different sizes. The area under the peak for each component will be proportional to the amount of that component present, so by measuring the peak areas you OOC_CH02_049-058.pdf 6 8/19/2013 11:15:17 AM Experiment 2 55 Preparation of Synthetic Banana Oil can estimate the percentage of each component in the distillate. This will tell you how much (if any) isopentyl alcohol and acetic acid remain in your product. This should be a green synthesis because it has a high atom economy, no organic solvents are used, and the catalyst reduces energy consumption and improves the yield. Acetic acid is expected to be slightly toxic to aquatic life, but it is a naturally occurring substance that readily breaks down to carbon dioxide and water in the environment. Isopentyl alcohol and isopentyl acetate aren’t considered to be serious environmental contaminants. The procedure for the synthesis of isopentyl acetate is summarized in the flow diagram in Figure 2.1, which illustrates the transformations or separations that occur during each operation. Appendix V tells you how to interpret such a flow diagram. Isopentyl acetate is known to be an alarm pheromone of the honeybee. A pheromone is a “molecular messenger” that produces a response, such as mating behavior or aggression toward a perceived threat, in another member of the same species. When a worker honeybee stings someone, it releases a tiny amount (about 1 mg) of isopentyl acetate in its stinger, which attracts more honeybees to the scene. Although isopentyl acetate alone doesn’t cause the bees to sting (other pheromones in the stinger do that), it agitates them and puts them on guard. So it might be wise to steer clear of beehives on your way home from the lab! Reactions and Properties O CH3C CH3 OH + HOCH2CH2CHCH3 acetic acid O H2SO4 CH3C isopentyl alcohol CH3 OCH2CH2CHCH3 + H2O isopentyl acetate Table 2.2 Physical properties acetic acid isopentyl alcohol isopentyl acetate sulfuric acid mol wt bp d Solubility 60.1 88.1 130.2 98.1 118 130 142 290 1.049 0.815 0.876 1.84 miscible 2.7 0.25 miscible Note: Boiling points are in °C; densities are in g/mL; solubilities are in g/100 mL water. DIRECTIONS Acetic acid causes chemical burns that can seriously damage skin and eyes; its vapors are highly irritating to the eyes and respiratory tract. Wear gloves, dispense under a hood, avoid contact, and do not breathe its vapors. Sulfuric acid causes chemical burns that can seriously damage skin and eyes. Wear gloves and avoid contact. Isopentyl alcohol and isopentyl acetate can irritate the skin, eyes, and respiratory tract. OOC_CH02_049-058.pdf 7 Safety Notes 2 2 0 1 2 acetic acid 3 W 1 sulfuric acid 8/19/2013 11:15:17 AM 56 Glacial acetic acid is a pure grade of acetic acid that freezes at about 17°C. Take Care! Wear gloves; avoid contact with acetic acid and sulfuric acid; do not breathe their vapors. Observe and Note: Look for and record any evidence of a chemical reaction. Stop and Think: What is the density of isopentyl acetate? Which layer should be on top, the aqueous layer or the organic layer? Take Care! Pressure may build up in the stoppered separatory funnel. Waste Disposal: Unless your instructor directs otherwise, dissolve the spent drying agent in the combined wash solvents and flush the mixture down the drain. Stop and Think: What does the boiling range of the distillate tell you about the purity of your product? Waste Disposal: Put any forerun and any residue left in the boiling flask into a designated waste container. Stop and Think: Can you guess which peak corresponds to which component based on the peak areas? Operational Organic Chemistry Reaction. Accurately weigh 150 mmol of isopentyl alcohol into a roundbottom flask of appropriate size and add boiling chips [OP-7b] or a magnetic stir bar [OP-10]. Under a hood, add 17 mL ( ' 300 mmol) of glacial acetic acid, and then carefully mix in 1.0 mL of concentrated sulfuric acid while stirring or swirling. Connect a condenser to the reaction flask, turn on the cooling water, start the stirrer (if you are using one), and heat the reaction mixture under reflux [OP-7c] for one hour after boiling begins. Separation. When the reaction time is up, allow the reaction mixture to cool nearly to room temperature. Turn off the cooling water and remove the reflux condenser. Transfer the reaction mixture to a separatory funnel, leaving the stir bar or boiling chips behind, and wash [OP-24] the mixture with 50 mL of water. Drain the aqueous layer and leave the organic layer in the separatory funnel. Then carefully wash the organic layer with two successive portions of 5% aqueous sodium bicarbonate, draining the aqueous layer after each washing. During the first washing, stir the layers until gas evolution subsides before you stopper the separatory funnel, and vent it frequently thereafter. Dry [OP-25] the crude isopentyl acetate with anhydrous sodium sulfate or magnesium sulfate. Purification and Analysis. Using standard-taper glassware [OP-2], assemble an apparatus for simple distillation [OP-30a]. Be sure the thermometer bulb is positioned as shown in Figure E7, OP-30, and have your instructor check your setup before you start. Distill the crude product, collecting any liquid that distills between 136°C and 143°C. Record the actual boiling range you observe; wait until the entire thermometer bulb is moist with condensing vapors, liquid is distilling into the receiver, and the temperature is stable before you record the initial temperature reading. Stop the distillation when only a drop or so of liquid remains in the boiling flask or when the temperature reaches 143°C. If the distillate is cloudy or contains water droplets, dry it [OP-25]. Weigh [OP-4] the distillate in a tared, labeled vial. Using a Carbowax column or another suitable column, obtain a gas chromatogram [OP-37] of the distillate and measure the peak areas as directed by your instructor. Unless your instructor indicates otherwise, assume that the components of the distillate appear on the gas chromatogram in the order (1) isopentyl alcohol, (2) isopentyl acetate, and (3) acetic acid. From the peak areas, calculate the percentage of each component in the distillate, then calculate your percent yield of isopentyl acetate based on its percentage in the distillate. Exercises 1. (a) Calculate the amount of isopentyl acetate that should be present in the reaction mixture at equilibrium, based on the quantities of starting materials you used and a value of 4.2 for the equilibrium constant. (Use the quadratic equation; because volumes cancel out, moles can be used in place of molar concentrations.) (b) Estimate the mass of isopentyl acetate that was lost (1) as a result of incomplete reaction, (2) during the washings, and (3) during the distillation (see OP-30a). Assume that the ester’s solubility in aqueous NaHCO3 is about the same as in water. OOC_CH02_049-058.pdf 8 8/19/2013 11:15:17 AM Experiment 2 2. 3. 4. 5. 6. 57 Preparation of Synthetic Banana Oil Compare the sum of these estimated losses with your actual product loss and try to account for any significant differences. What gas escaped during the sodium bicarbonate washing? Write balanced equations for two reactions that took place during this operation. (a) Calculate the atom economy and reaction efficiency of your synthesis of isopentyl acetate. (b) Tell how the procedure for the preparation of isopentyl acetate might be modified to increase the reaction efficiency. (c) Describe some green features of your synthesis, and any that aren’t so green. Describe and explain how each of the following experimental errors or variations might affect your results. (a) You failed to dry the reaction flask after washing it with water. (b) You forgot to add the sulfuric acid. (c) You used twice the amount of acetic acid specified in the procedure. (d) You left out the sodium bicarbonate washing step. (e) Your thermometer bulb was 1 cm higher than it should have been. (a) In the “Understanding the Experiment” section, it was stated that the reaction of an equimolar mixture of isopentyl alcohol and acetic acid will produce, at most, 67% of the theoretical amount of isopentyl acetate. Verify this with an equilibrium constant calculation, using K = 4.2. (b) Compare this with the corresponding percentage for the conditions used in this experiment (see Exercise 1). Are your results consistent with Le Châtelier’s principle? Explain. Based on the procedure that you used in this experiment and using the same molar quantities of reactants, develop a procedure that would be suitable for the preparation of isobutyl propionate. Specify the amounts of all materials required and a distillation range for the product. Obtain the necessary physical properties from one of the reference books listed in the Bibliography. Isopentyl acetate equilibrium i-PtOH + HOAc i-PtOAc K= + H2O [i-PtOAc][H2O] = 4.2 [i-PtOH][HOAc] Ac = acetyl, CH3CO i-Pt = isopentyl, CH3CHCH2CH2 CH3 O CH3CH2C CH3 OCH2CHCH3 isobutyl propionate Other Things You Can Do (Starred items require your instructor’s permission.) *1. You and your coworkers can prepare a series of esters and compare their odors. *2. Prepare another ester of a primary alcohol, such as butyl acetate or isobutyl propionate, by the general method described for isopentyl acetate. Work out a procedure for the synthesis (see Exercise 6) and have it approved by your instructor. In some cases, a longer reflux time may be necessary for satisfactory results. 3. Read about the isolation of isopentyl acetate (isoamyl acetate) in the alarm pheromone of the honeybee in Nature 1962, 195, 1018. 4. Read about various types of pheromones in Chemical Communication: The Language of Pheromones [Bibliography, L3]. OOC_CH02_049-058.pdf 9 8/19/2013 11:15:17 AM OOC_CH02_049-058.pdf 10 8/19/2013 11:15:17 AM


![Isopentyl Acetate [Banana Oil] In this experiment, we prepare an](http://s3.studylib.net/store/data/008730431_1-27d60b2e4c34d7ce18e86c4d6c41e860-300x300.png)
