EXPERIMENT 5: USING QUALITATIVE ANALYSIS TO IDENTIFY IONS
advertisement

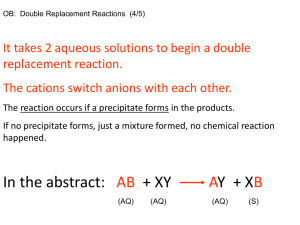
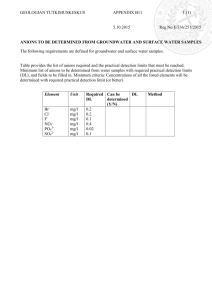
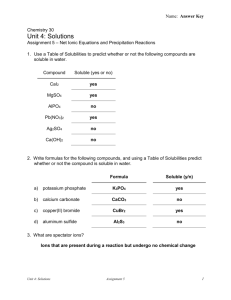
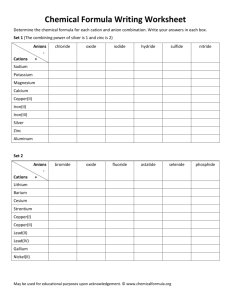
EXPERIMENT 5: USING QUALITATIVE ANALYSIS TO IDENTIFY IONS Objectives 1. Identify some commonly occurring ions using qualitative tests. 2. Write equations used for their identification. CAUTION Do not get silver nitrate (AgNO3) on your skin. It will leave a dark brown-black stain that may take a week to fade. It will permanently stain clothing and paper. Materials and Equipment Small test tubes, disposable pipettes, marking crayons and safety glasses plus the following reagents – Part 1. Anions Solutions containing the following anions to be tested: Carbonate, CO3- (from 0.1 M Na2CO3) Chloride, Cl- (from 0.1 M NaCl) Phosphate, PO43- (from 0.1 M Na3PO4) Sulfate, SO42- (from 0.1 M Na2SO4) Reagents to be used for testing the preceding anions: Dilute hydrochloric acid, 6 M HCl Dilute nitric acid, 6 M HNO3 Silver nitrate solution, 0.1 M AgNO3 Ammonia water, 6M NH3 (aq) Ammonium molybdate solution, 0.2 M (NH4)2MoO4 Barium chloride solution, 0.5 M BaCl2 Part 2. Cations Solutions containing the following cations to be tested: Ammonium, NH4+ (from 0.5 M NH4NO3) Boron, B3+ (from 1.0 M H3BO3 in 1M HCl) Calcium, Ca2+ (from 0.5 M Ca(NO3)2) Strontium, Sr+ (from 0.5 M SrNO3) Reagents to be used for testing the preceding cations: Dilute hydrochloric acid, 6 M HCl Aqueous ammonia, 6 M NH3 (aq) Sodium hydroxide, 2 M NaOH Ammonium oxalate, 1 M (NH4)2C2O4 Proper Waste Disposal: Any solution or solid mixture that contains Ag (silver), Ba (barium), Mo (molybdenum) or Sr (strontium) must be disposed of in the heavy metals waste container. Introduction A branch of chemistry that is concerned with the identification of chemical substances is qualitative analysis. Through qualitative analysis you can determine whether or not a material is present or absent, but you won’t necessarily be able to tell how much of that material there is in a sample. In Part 1 of this experiment, you will learn to identify some of the common anions (negative ions) frequently encountered in the laboratory by testing the indicated solutions: Carbonate, CO3- (from 0.1 M Na2CO3) Chloride, Cl- (from 0.1 M NaCl) Phosphate, PO43- (from 0.1 M Na3PO4) Sulfate, SO42- (from 0.1 M Na2SO4) When AgNO3 is added to the solutions to be tested for anions, several insoluble salts (precipitates) form. However, when nitric acid is added to these precipitates, all the solids except AgCl will dissolve. The insoluble AgCl will remain in the test tube. Phosphate ion (PO43-) reacts with ammonium molybdate to form a yellow precipitate upon heating. When barium chloride, BaCl2, is added to a solution containing SO42-, a precipitate of BaSO4 forms. Barium may form insoluble salts with some other anions, but the addition of HNO3 dissolves all barium salts except BaSO4. The insoluble BaSO4 remains in the test tube after HNO3 is added. Carbonate anion (CO32-) is identified by adding HCl, which produces bubbles of CO2 gas. The net ionic equation for the carbonate anion is: 2 H+ + CO32- → CO2 + H2O After studying the specific confirmatory tests for the known anion solutions, you will be asked to deduce the identity of an unknown anion in solution. Each confirmatory test is specific and gives characteristic results for only one anion. In Part 2 of this experiment, you will observe the tests for the following cations contained in the indicated solutions: Ammonium, NH4+ (from 0.1 M NH4NO3) Boron, B3+ (from 1.0 M H3BO3 in 1M HCl) Calcium, Ca2+ (from 0.1 M Ca(NO3)2) Strontium, Sr+ (from 0.1 M SrNO3) The presence of B3+, Sr+, and Ca+2 can quickly be determined by the distinctive flame color the ions give in flame tests. Additional chemical tests will also be run, similar to the procedures we followed for anions. The calcium ion Ca2+reacts with ammonium oxalate, NH4)2C2O4, to give a white precipitate. When ammonium, NH4+ is converted to ammonia (NH3), the characteristic odor of ammonia is detected, and the NH3 vapor will turn red litmus paper to blue. After studying the flame tests and then carrying out specific confirmatory tests for the known cation solutions, you will be asked to deduce the identity of an unknown cation in solution. Each confirmatory test is specific and gives characteristic results for only one cation. Procedure Reminder: Be careful in selecting the reagent solutions – make certain you are using the right bottle. Some of the solutions have very similar names, so read the label twice! To avoid cross-contamination, do not touch the pipette tips to any surface. Use the crayons for marking your test tubes. Part 1. Anions ClTo 2 mL of the Cl- solution add 0.5 mL of silver nitrate, 0.1 M AgNO3 solution. A white precipitate that easily dissolves when the solution is made basic with aqueous ammonia, 6 M NH3 (aq) solution indicates the presence of the chloride ion. Hint: test a drop of your solution with red litmus paper. If it turns blue, the solution is basic. Report the procedure with your unknown solution. Record your observations. (Note: Dispose solutions into heavy metals waste container). SO42Add 1 mL of 1 M BaCl2 solution to 2 mL of the SO42- solution. A white precipitate that does not dissolve when HCl is added indicates the presence of sulfate. Repeat the procedure with your unknown solution. Record your observations. (Note: Dispose solutions into heavy metals waste container). PO43Add dilute nitric acid, 6 M HNO3 to 2 mL of the PO43- solution until it tests acid with blue litmus paper. Add 1 mL of ammonium molybdate, 0.2 M (NH4)2MoO4 solution. Gentle warming over a Bunsen burner may be necessary to produce a yellow precipitate. Repeat the procedure with your unknown solution. Record your results. . (Note: Dispose solutions into heavy metals waste container). CO32Add dilute hydrochloric acid (6 M HCl) to 5 mL of the CO32- solution until it tests acid with blue litmus paper. Carbonates produce an odorless gas. If you see bubbles and there is no odor, this indicates the presence of carbonate. Repeat the procedure with your unknown solution. Record your observations. Part 2. Cations B3+, Sr+, Ca2+ Flame Tests Obtain several wooden splints (coffee stirrers). Place several drops on B3+ solution on one splint; hold the treated end of the splint in the non-luminous flame of your Tirrill or Bunsen burner. Make a note of the color of the flame produced by the B3+ ion. Repeat this process with the Sr+, Ca2+ solutions and your unknown solution. For your unknown solution, if the color matches the color of one of the three ion solutions you test, you can conclude you have one of the ions B3+, Sr+, Ca2+in your sample. Cation Specific Tests There may be times when it is difficult to distinguish the difference in the flame colors produced by cations, or there may be no color produced at all. In these cases, there are tests that can be conducted that give specific results for certain ions. You will perform two such tests. Ca2+ Calcium ion Ca2+ can also be detected by a chemical test. Place 2 mL of Ca2+ solution in a small test tube. Add a few drops of ammonium oxalate, 1 M (NH4)2C2O4 solution to each solution to be tested. Look for a cloudy white precipitate as a positive test for the presence of Ca2+ ion. Repeat the test with your unknown solution. Record your results. NH4+ Put about 2 mL of the NH4+ solution to be tested into a small test tube and make the solution basic with sodium hydroxide (2 M NaOH) solution. Hint: see if red litmus paper turns blue to test if you have added enough sodium hydroxide. Warm the mixture gently – do not boil. Test for ammonia fumes by holding a piece of moist red litmus paper just above the mouth of the test tube while you are warming the solution. Be careful not to touch the litmus paper on the test tube or you may get erroneous results. Repeat the procedure with your unknown solution. Record your observations. Observations and Data Fill out the report sheets at the end of this experiment. Questions: Answer the questions at the end of the report sheets. Additional Resources Check Appendices G, H & M for additional resources for this experiment.