Practice with Properties of Metals, nonmetals, + ionic
advertisement

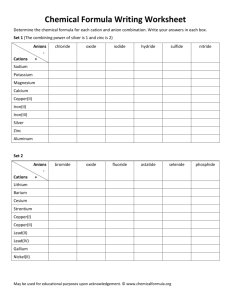
OB: to review the properties of metals, nonmetals, ionic compounds, and then… Polyatomic Ions Take out your reference tables and some paper for some notes. Define the Properties… Luster Ductile Malleable Conducts electricity Conducts heat Phase at room temp Forms Cations? Forms Anions? Relative Melting point Particle (atom, molecule, FU) Property Luster Ductile malleable Conducts electricity Conducts heat Phase at room temp Forms cations? Forms anions? Relative melting point particles Metals Nonmetals Ionic compounds Property Metals Nonmetals Ionic compounds Luster Yes No No Ductile Yes No No Malleable Yes No No Conducts electricity Yes No No Conducts heat Yes No No Phase at room temp Solid Solid, liquid, gas Solid Forms cations? Yes No X Forms anions? No Yes X Relative melting point High Lowest Highest Particles Atoms Atoms or diatomic molecules Formula units All of our polyatomic ions will be found on table E on the front page of the reference tables They are multiple groups of atoms, acting as a single unit, with a charge, as an ION. Most are anions, but the first few are cations. Ammonium is common, the other 2 cations are much less common. The anions are all equally common in our course. Never change their names, ever. Some are similar in formula (sulfite and sulfate) but very different in name. Be careful. Lots have fun names to say out loud. Let’s say some now. When naming the ionic compounds, we always say the cation first, then the anion. These polyatomics can readily combine with the monoatomic ions from the periodic table. Cations Anions Li+1 CO3-2 Mg+2 ClO3-1 Compound formula CaCrO4 SrCr2O7 NH4+1 OH-1 Names Cations Anions Compound formula Names Li+1 CO3-2 LiCO3 Lithium carbonate Mg+2 ClO3-1 MgClO3 Magnesium chlorate Ca+2 CrO4-2 CaCrO4 Calcium chromate Sr+2 Cr2O7-2 SrCr2O7 Strontium dichromate NH4+1 OH-1 NH4OH Ammonium hydroxide Cations Anions Compound formula Names Ammonium oxalate Ammonium Phosphide Cesium sulfate Cesium sulfite Barium thiocyanate Cations NH4 Anions +1 C2O4 NH4+1 P-3 -2 Compound formula Names (NH4)2C2O4 Ammonium oxalate (NH4)3P3 Ammonium Phosphide Cs+1 SO4 -3 Cs2SO4 Cesium sulfate Cs+1 SO3-3 Cs2SO3 Cesium sulfite Ba+2 SCN-1 Ba(SCN)2 Barium thiocyanate Cations Anions Compound formula (NH4) 2SO4 NH4HCO3 NaCN KOH Ba(SCN) 2 Names Cations NH4 +1 Anions SO4 -3 Compound formula Names (NH4) 2SO4 Ammonium sulfate NH4+1 HCO3-1 NH4HCO3 Ammonium hydrogen carbonate Na+1 CN-1 NaCN Sodium cyanide K+1 OH-1 KOH Potassium hydroxide Ba+2 SCN-1 Ba(SCN) 2 Barium thiocyanate Cations Anions Na+1 C2H3O2-1 Ca+2 MnO4-1 Sr+2 O2-2 Al+3 MnO4-1 Be+2 PO4-3 Compound formula Names Cations Anions Compound formula Names Na+1 C2H3O2-1 NaC2H3O2 Sodium acetate Ca+2 MnO4-1 Ca(MnO4)2 Calcium permanganate SrO2 Strontium peroxide Al(MnO4)3 Aluminum permanganate Be3(PO4)2 Beryllium phosphate Sr+2 Al+3 Be+2 O2 -2 MnO4-1 PO4 -3