STRATOS system for chest wall reconstruction
A Novel Titanium Rib Bridge
System for Chest Wall
Reconstruction
Aman S. Coonar, MD, FRCS(CTh),
Nagmi Qureshi, FRCR, Ian Smith, FRCP,
Francis C. Wells, FRCS, Erhard Reisberg, MBA, and
Jean-Marie Wihlm, MD
Departments of Thoracic Surgery, Radiology, and Chest
Medicine, Papworth Hospital, Cambridge, United Kingdom;
Department of Thoracic Surgery, Hôpitaux Universitaires de
Strasbourg, Strasbourg, France; MedXpert, Heitersheim,
Germany
Chest wall resection for liposarcoma was performed. To reconstruct the chest wall we used a novel titanium rib bridge system and preserved anatomically equivalent layers.
(Ann Thorac Surg 2009;87:e46 – 8)
© 2009 by The Society of Thoracic Surgeons
R estoring individual rib continuity may be beneficial in that more normal physiology may be maintained.
Titanium has advantages including a high strength to weight ratio, osseointegration, and causes less interference with computed tomography than higher density metals. A reduced rate of computed tomographic artefacts allows more accurate three-dimensional reconstructions. Titanium is nonferromagnetic, so it can be used safely with magnetic resonance imaging. The patient gave informed consent for the operation and subsequent report.
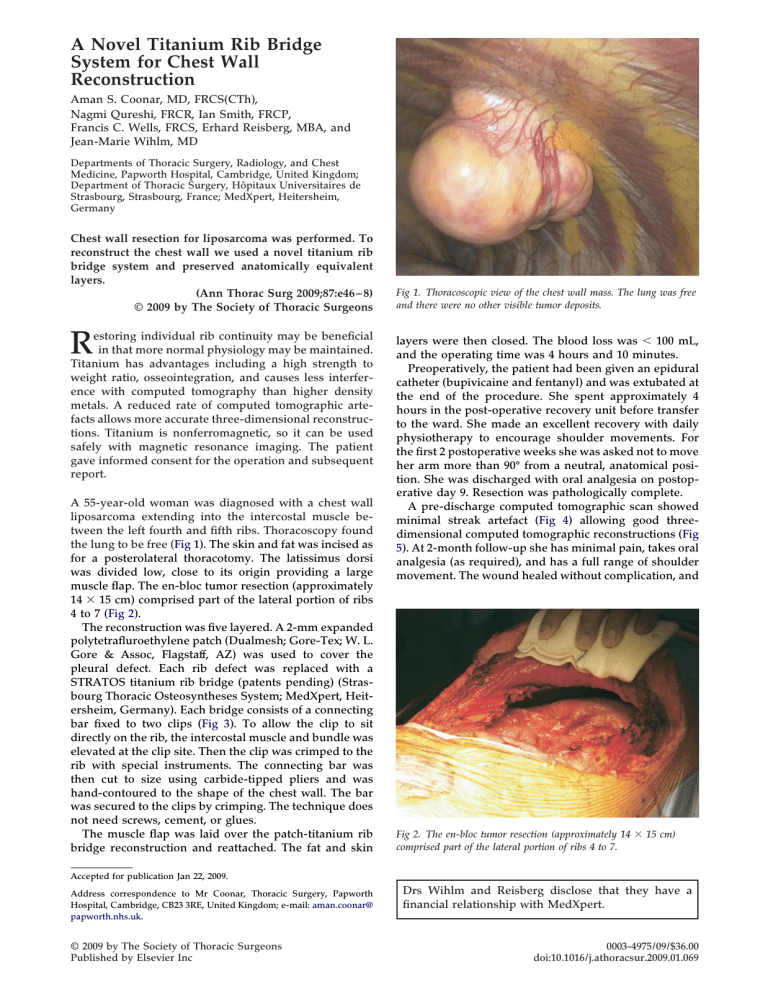
A 55-year-old woman was diagnosed with a chest wall liposarcoma extending into the intercostal muscle between the left fourth and fifth ribs. Thoracoscopy found
the lung to be free ( Fig 1 ). The skin and fat was incised as
for a posterolateral thoracotomy. The latissimus dorsi was divided low, close to its origin providing a large muscle flap. The en-bloc tumor resection (approximately
14 ⫻ 15 cm) comprised part of the lateral portion of ribs
The reconstruction was five layered. A 2-mm expanded polytetrafluroethylene patch (Dualmesh; Gore-Tex; W. L.
Gore & Assoc, Flagstaff, AZ) was used to cover the pleural defect. Each rib defect was replaced with a
STRATOS titanium rib bridge (patents pending) (Strasbourg Thoracic Osteosyntheses System; MedXpert, Heitersheim, Germany). Each bridge consists of a connecting
bar fixed to two clips ( Fig 3 ). To allow the clip to sit
directly on the rib, the intercostal muscle and bundle was elevated at the clip site. Then the clip was crimped to the rib with special instruments. The connecting bar was then cut to size using carbide-tipped pliers and was hand-contoured to the shape of the chest wall. The bar was secured to the clips by crimping. The technique does not need screws, cement, or glues.
The muscle flap was laid over the patch-titanium rib bridge reconstruction and reattached. The fat and skin
Accepted for publication Jan 22, 2009.
Address correspondence to Mr Coonar, Thoracic Surgery, Papworth
Hospital, Cambridge, CB23 3RE, United Kingdom; e-mail: aman.coonar@ papworth.nhs.uk
.
© 2009 by The Society of Thoracic Surgeons
Published by Elsevier Inc
Fig 1. Thoracoscopic view of the chest wall mass. The lung was free and there were no other visible tumor deposits.
layers were then closed. The blood loss was ⬍ 100 mL, and the operating time was 4 hours and 10 minutes.
Preoperatively, the patient had been given an epidural catheter (bupivicaine and fentanyl) and was extubated at the end of the procedure. She spent approximately 4 hours in the post-operative recovery unit before transfer to the ward. She made an excellent recovery with daily physiotherapy to encourage shoulder movements. For the first 2 postoperative weeks she was asked not to move her arm more than 90° from a neutral, anatomical position. She was discharged with oral analgesia on postoperative day 9. Resection was pathologically complete.
A pre-discharge computed tomographic scan showed
5 ). At 2-month follow-up she has minimal pain, takes oral
analgesia (as required), and has a full range of shoulder movement. The wound healed without complication, and
Fig 2. The en-bloc tumor resection (approximately 14 ⫻ 15 cm) comprised part of the lateral portion of ribs 4 to 7.
Drs Wihlm and Reisberg disclose that they have a financial relationship with MedXpert.
0003-4975/09/$36.00
doi:10.1016/j.athoracsur.2009.01.069
Ann Thorac Surg
2009;87:e46 – 8
CASE REPORT COONAR ET AL
NOVEL TITANIUM RIB BRIDGE SYSTEM e47
Fig 3. Illustrative model of rib defect reconstruction with STRATOS rib bridges (patents pending) (Strasbourg Thoracic Osteosyntheses
System [MedXpert, Germany]). Each bridge consists of 2 clips and 1 connecting bar crimped to each other and the rib.
there is no evidence of the implant loosening or of migration.
The use of a patch and good muscle coverage prevented herniation. Dividing the muscle very low allowed most of the muscle to remain functional. This titanium rib bridge system allows individual rib continuity to be maintained and may allow more physiological “bucket handle” rib movements than larger fixed prosthesis such as mesh and methylmethacrylate cement.
The use of titanium as a biological prosthesis is well established. Titanium rapidly forms an oxide layer that is highly corrosion-resistant. It has the highest strength-toweight ratio of any metal. Titanium can integrate with bone (osseointegration)
[1] . This is reliant on osteoblasts
attaching to the titanium surface eventually forming mineralized bone in continuity with the implant, which then is less likely to “loosen” over time.
Another advantage of titanium in comparison with
Fig 5. Three-dimensional computed tomographic reconstruction showing the titanium rib bridges maintaining continuity of the ribs.
some other metals is its relative noninterference with cross-sectional imaging
artefacts than steel, because it is less dense; this is important, because reduced artefacts permit more accurate three-dimensional reconstruction, which may be important in subsequent clinical management. Also, titanium is nonferromagnetic, allowing patients to be examined with magnetic resonance imaging.
The system has a limited number of parts and can be tailored on the operating table to the needs of a given operation
[3] . An alternative may be preoperative be-
spoke implant manufacture. However, this reduces adaptability at the time of surgery and currently the costs would be prohibitive. In the future, this may be modified by the developing technology of computer-assisted manufacturing using three-dimensional printing and tissue engineering. Other emerging methods to reconstruct the chest wall include the distraction osteogenesis
the use of materials that encourage new bone growth or osseointegration and complex tissue engineering.
This is a single report using a novel system. Future comparative studies that include cost analyses may be helpful in determining indications for use. The development and introduction of new technology is often expensive, but may be justified if associated with better functional outcomes.
Fig 4. Computed tomographic scan on postoperative day 8 shows minimal streak artefact despite four titanium rib bridges. The polytetrafluroethylene patch and a small pleural effusion is also visible.
The surgery was performed by Mr Coonar at Papworth Hospital.
The cost of the implants and imaging was covered by the United
Kingdom National Health Service.
e48 CASE REPORT COONAR ET AL
NOVEL TITANIUM RIB BRIDGE SYSTEM
Ann Thorac Surg
2009;87:e46 – 8
References
1. Branemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Scand J Plast
Reconstr Surg Suppl 1977;16:1–132.
2. Mi-Jung Lee, Sungjun Kim, Sung-Ah Lee, et al. Overcoming artifacts from metallic orthopedic implants at high-field-strength mr imaging and multi-detector CT. Radiographics 2007;27:791–803.
3. Wihlm JM, Grosdidier G, Chapelier A. Thoracic osteosyntheses for chest wall malformations, traumas and tumors using the STRATOS (TM) titanium system. ICVTS 2007;6(Suppl
3)S273.
4. Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater 2008;15:53–76.








