ELECTROCHEMICAL THERMODYNAMICS AND ELECTRODE
advertisement

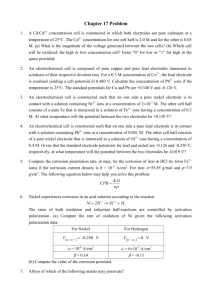
ELECTROCHEMICAL THERMODYNAMICS AND ELECTRODE POTENTIAL Reading Material: Chapter 2 in “Principles and Prevention of Corrosion, Denny Jones, Prentice-Hall, 1996”. Dr. Ramazan Kahraman Chemical Engineering Department King Fahd University of Petroleum & Minerals Dhahran, Saudi Arabia 1 ELECTROCHEMISTRY ● Deals with chemical changes produced by an electric current and with the production of electricity by chemical reactions ● All electrochemical reactions involve transfer of electrons and are redox (oxidation-reduction) reactions ● Electrochemical reactions take place in electrochemical cell (an apparatus that allows a reaction to occur through an external conductor) 2 ELECTROCHEMICAL CELLS Two types: 1. Electrolytic cells: - these are cells in which an external electrical source forces a nonspontaneous reaction to occur (one common process is called electrolysis) (not to be covered here) 2. Voltaic cells: - also called galvanic cells. In these cells spontaneous chemical reactions generate electrical energy and supply it to an external circuit (corrosion and fuel cells are examples) 3 ELECTRODES (as also covered earlier) ● Electric current enters and exits the cell by electrodes electrodes are surfaces upon which oxidation or reduction half-reactions occur ● Two kinds of electrodes: ▬ Cathode: - electrode at which reduction occurs (electrons are gained by a species) ▬ Anode: - electrode at which oxidation occurs (as electrons are lost by some species) 4 GALVANIC CELLS ● Cells in which spontaneous reactions produces electrical energy ● The two half-cells are separated so that electron transfer occurs through an external circuit ● Each half-cell contains the oxidized and reduced forms of species in contact with each other ● Half-cells linked by a piece of wire and a salt bridge 5 + 6 A salt bridge has three functions 1. It allows electrical contact between the two halfcells 2. It prevents mixing of the electrode solutions 3. It maintains electrical neutrality in each half-cell as ions flow into and out of the salt bridge Note that: No electron flow between the two electrodes in separate solutions without the salt bridge. 7 In galvanic cells, voltage/potential difference between the electrodes drops to 0 as the reaction proceeds Salt bridge Zn Zn 2+ (1.0 M) Cu 2+ (1.0 M) Cu Electrode Species (with concentrations) in contact with electrodes 8 The Silver-Copper Cell • Composed of two half-cells: 1. A strip of copper immersed in 1 M CuSO4 2. A strip of silver immersed in 1 M AgNO3 • Experimentally we see: - Initial voltage (potential difference between electrodes when not connected yet) is 0.46 volts - When electrodes connected - The mass of the copper electrode decreases - The mass of the silver electrode increases - [Cu2+] increases and [Ag+] decreases 9 The Silver-Copper Cell (cont.) Cu → Cu2+ + 2e- (oxidation, anode) 2(Ag+ + e- → Ag) (reduction, cathode) 2Ag+ + Cu → Cu2+ + Ag (overall cell reaction) Cu |Cu2+(1.0 M) ||Ag+(1.0 M) | Ag • Notice that in this case the copper electrode is the anode 10 FREE ENERGY and ELECTRODE POTENTIAL • Associated with each galvanic cell is a potential difference between the electrodes called the equilibrium cell potential, Ecell (also called reversible or electrochemical potential or electromotive force – emf) E = Ecathode - Eanode The electrodes are considered not connected to each other so no reaction takes place on them yet, i.e. each electrode is at equilibrium with its environment • E measures the spontaneity of the cell’s redox reaction ∆G = -nFEcell • Higher (more positive) cell potentials (more negative ∆G) indicate a greater driving force for the reaction as written (in forward direction, i.e. from left to right) 11 STANDARD ELECTRODE POTENTIAL • The standard equilibrium cell potential (E°cell) is for the cell operating under standard state conditions • For an electrochemical cell, standard conditions are: - solutes at 1 M concentrations - gases at 1 atm partial pressure - solids and liquids in pure form - all at some specified temperature, usually 298 K 12 The electrode potentials are commonly measured versus the Standard Hydrogen Electrode (SHE): E° = 0.00 V EoZn= -0.762 vs SHE Zn and Pt electrodes are at equilibrium with their environments at standard conditions and they are are not connected to eachother by a conductor so no current flows between Zn and Pt. The potential measured (EoZn) is the equilbrium potential at standard conditions vs SHE. 13 EQUILIBRIUM POTENTIAL When we immerse a metal in solution, there will be a tendency for the metal to react with the solution, either with metal atoms dissolving as cations or cations already in the solution depositing as metal atoms: Zn → Zn2+ + 2eZn2+ + 2e- → Zn As a result of these reactions, the metal will tend to accumulate a negative or positive charge. The build-up of this charge on the metal will change its potential in such a way as to inhibit the reaction generating the charge until the potential reaches a value at which the rates of the two reactions are equal and opposite. This is known as the equilibrium potential, and is the potential the metal will adopt in the solution in the absence of any other reactions. 14 15 STANDARD ELECTRODE POTENTIAL (cont.) • For covenience all half-cell reactions are written in the table in reduction form and the electrode potentials are invariant e.g. Zn=Zn2++2e- and Zn2++2e-=Zn are identical and represent zinc in equilibrium with its ions with a potential of -0.762 V vs SHE • The more positive the E° value for a half-reaction the greater the tendency for the reaction to proceed as written (in reduction-cathodic form) at standard conditions • The more negative the E° value, the more likely is the reverse of the reaction as written (in oxidation-anodic form) at standard conditions • However, usually concentrations of reactants differ from one another and also change during the course of a reaction, in that case E°cell and the actual Ecell are related by the Nernst Equation 16 THE NERNST EQUATION E = E° - (RT/nF) lnK E = equilibrium potential under the nonstandard conditions E° = equilibrium potential at standard state (all reactants and products at unit activity) R = gas constant, 8.314 J/mol.K T = absolute temperature n = number of equivalents (moles of electrons) transferred F = Faraday’s constant, 96,485 J/V ⋅ equivalent ⋅ mol (= C/equivalent ⋅ mol) K= ∏ activities of products raised to the power of their coefficients activities of reactants raised to the power of their coefficients p.s. If the equation is to be used for determining the equilibrium potential for a half cell rxn, then the half cell rxn is to be written in reduction form or the (-) sign in the equation is to be replaced by (+) sign in case the half cell rxn is written in oxidation form. 17 Notation: E for the overall cell rxn, e for the half-cell rxns p.s. If the equation is to be used for determining the equilibrium potential for a half cell rxn, then the half cell rxn is to be written in reduction form or the (-) sign in the equation is to be replaced by (+) sign in case the half cell rxn is written in oxidation form. 18 ACTIVITY • Activity of a dissolved species A (A) is equal to its concentration in moles per 1000 grams of water (molality) multiplied by the activity coefficient, f. Activity coefficients are extensively tabulated in numerous chemical and electrochemical handbooks. • Activity of a gas is approximated at ordinary pressures by its partial pressure in atmospheres (atm). • The activities of pure solids and water are set equal to unity in aquaeous solutions. • At 25°C, 2.303RT/F = 0.0592 V ⋅ equivalent 19 ACTIVITY (Cont.) Activity Coefficients of Strong Electrolytes (M=molality) [“Corrosion and Corrosion Control”, H. H. Uhlig and R. W. Revie, John Wiley & Sons, 1985.] 20 ACTIVITY (Cont.) Activity Coefficients of Strong Electrolytes (M=molality) [“Corrosion and Corrosion Control”, H. H. Uhlig and R. W. Revie, John Wiley & Sons, 1985.] 21 ACTIVITY (Cont.) Activity Coefficients of Strong Electrolytes (M=molality) [“Corrosion and Corrosion Control”, H. H. Uhlig and R. W. Revie, John Wiley & Sons, 1985.] 22 PREDICTION OF SPONTANEITY 1. First write the half-cell rxn with the more positive (less negative) E° for the reduction-cathodic along with its half cell electrode potential 2. Write the other half-cell rxn as an oxidation-anodic and include its half cell electrode potential 3. Balance the electron transfer 4. Obtain the cell rxn by adding the reduction and oxidation half-cell rxns. 5. Determine the overall cell potential, Ecell, from Ecell= Ecathode- Eanode 23 Note that half-cell reaction potentials are the same regardless of the species’ stoichiometric coefficient in the balanced equation. Ecell > 0 Forward reaction (left-to-right) is spontaneous Ecell < 0 Backward reaction (right-to-left) is spontaneous 24 EXAMPLE PROBLEM 1 Notation: e for the half-cell rxns, E for the overall cell rxn. 25 EXAMPLE BROBLEM 2 Notation: E for the overall cell rxn, e for the half-cell rxns 26 27 EXAMPLE PROBLEM 3 Notation: e for the half-cell rxns, E for the overall cell rxn. Assuming standard states for all reactants and products, determine the spontaneous direction of the following reactions by calculating the cell potential: CuCl2 + H2 = Cu + 2HCl CuCl2 + H2 Æ Cu + 2HCl 28 A NOTE FOR THE SIGN NOTATION Note that the sign notation used in the text is different and confusing. The text uses E = ec + ea which is actually identical to the notation presented above since the text takes ea as the negative value of the anode half-cell electrode potential. 29 SECONDARY REFERENCE ELECTRODES • If a reference electrode other than SHE is used to measure the equilibrium potential of a reaction, the potential of the reference electrode relative to SHE should be added to the measured potential if one wants to determine the equilibrium potential of the reaction relative to SHE • The reference electrodes are also used to measure the corrosion potential of a corrosion cell (Ecorr) (to be covered later) 30 Potential Values for Common Secondary Reference Electrodes (Standard Hydrogen Electrode included for reference) 31 EXAMPLE PROBLEM (Prb. 2.15 in “Principles and Prevention of Corrosion”, Denny Jones, 1996) A corrosion potential of -0.229 V versus SCE was measured for a corroding alloy. What is the potential versus (a) SHE, (b) Ag/AgCl (saturated), (c) Cu/saturated CuSO4? (a) (b) (c) Home Exercise 32 Potential The Pourbaix (Equilibrium Potential-pH) Diagram 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 O2 is stable 2H2O = O2 + 4H+ + 4e- H2O is stable 2H+ + 2e- = H2 H2 is stable 0 7 14 33 Potential Pourbaix Diagram for Zinc 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 Equilibrium for Zn2+ + 2OH- ⇔ Zn(OH)2 ZnO22stable in solution Zn(OH)2 stable solid Zn2+ stable in solution Equilibrium for Zn + 2OH- ⇔ Zn(OH)2 + 2eEquilibrium for Zn + 4OH- ⇔ ZnO22- + 2H2O + 2e- Zn metal stable 0 Equilibrium for Zn(OH)2 + 2OH- ⇔ ZnO22- + 2H2O 7 14 Equilibrium for Zn ⇔ Zn2+ + 2e- 34 Pourbaix Diagram for Zinc Potential Corrosion requires strong oxidising agent 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 Corrosion requires strong oxidising agent Corrosion Passivity Corrosion Corrosion is possible, but likely to be stifled by solid corrosion product Corrosion possible with oxygen reduction Corrosion possible with hydrogen evolution Immunity 0 7 14 Corrosion is thermodynamically impossible 35 2H+ + 2e- → H2 hydrogen evolution in acids 2H2O + 2e- → H2 + 2OH- hydrogen evolution in water/bases (the above two reactions are equivalent reactions) O2 + 2H2O + 4e- → 4OH- oxygen reduction in water/bases O2 + 4H+ + 4e- → 2H2O oxygen reduction in acids (the above two reactions are equivalent reactions) 36 Potential Pourbaix Diagram for Gold 2.0 1.6 C Passivity 1.2 0.8 0.4 Gold metal stable 0.0 -0.4 Immunity region -0.8 -1.2 -1.6 0 7 Gold can’t corrode with oxygen reduction or hydrogen evolution C 14 37 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 Cu oxides stable Cu2+ stable in solution CuO22- stable in soln. Potential Pourbaix Diagram for Copper No - hydrogen evolution only occurs below the potential for copper corrosion Cu metal stable 0 7 Will copper corrode in deaerated acid? 14 38 2.0 1.6 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 Cu oxides stable Cu2+ stable in solution CuO22- stable in soln. Potential Pourbaix Diagram for Copper (Cont.) Usually it will just passivate, but corrosion can occur in slightly acid solutions Cu metal stable 0 7 Will copper corrode in neutral aerated waters? 14 39 Potential Pourbaix Diagram for Iron 2.0 1.6 1.2 Fe3+ 0.8 0.4 Fe oxides stable 0.0 -0.4 Fe2+ stable -0.8 Fe metal stable -1.2 -1.6 0 7 Will iron corrode in deaerated acid? 14 Yes - there is a reasonably wide range of potentials where hydrogen can be evolved and iron dissolved 40 Potential Pourbaix Diagram for Iron 2.0 1.6 1.2 Fe3+ 0.8 0.4 Fe oxides stable 0.0 -0.4 Fe2+ stable -0.8 Fe metal stable -1.2 -1.6 0 7 Will iron corrode in neutral waters? Yes - although iron can form an oxide in neutral solution, it tends not to form directly on the metal, as the potential is too low, therefore it is not protective. 14 41 Potential Pourbaix Diagram for Iron 2.0 1.6 1.2 Fe3+ 0.8 0.4 Fe oxides stable 0.0 -0.4 Fe2+ stable -0.8 Fe metal stable -1.2 -1.6 0 7 Will iron corrode in alkaline solutions? No - iron forms a solid oxide at all potentials, and will passivate 14 42 Potential Pourbaix Diagram for Aluminum 1.2 0.8 0.4 0.0 -0.4 -0.8 -1.2 -1.6 -2.0 -2.4 Al3+ Al2O3 AlO2- Al 0 7 14 43 Limitations of Pourbaix Diagrams z Tell us what can happen, not necessarily what will happen z No information on rate of reaction z Can only be plotted for pure metals and simple solutions, not for alloys 44 EXAMPLE PROBLEM (Prb. 2.10 in “Principles and Prevention of Corrosion”, Denny Jones, 1996) Using the Pourbaix diagram for nickel, give the anodic and cathodic reactions on Ni in water for the following conditions, assuming activity of 10-6 for all soluble species: (a) deaerated pH 2, (b) deaerated pH 10, (c) aerated pH 2, aerated pH 10. 45 46 Home Work Problems z Determine whether silver will corrode with hydrogen evolution (PH2=1 atm) in deaerated KCN solution, pH=9, when CN- activity=1.0 and Ag(CN)2 activity=0.001. What is the cell potential in volts? Ag(CN)2- + e- ↔ Ag + 2CN- z eo=-0.31 V Prbs. 3, 5, 6 of Chapter 2 in “Principles and Prevention of Corrosion”, Denny Jones, Prentice-Hall, 1996. 47 Home Exercise Problems z Prbs. 7, 8, 11 and 12 of Chapter 2 in “Principles and Prevention of Corrosion”, Denny Jones, Prentice-Hall, 1996. 48 References z “Principles and Prevention of Corrosion”, Denny Jones, Prentice-Hall, 1996. z “Corrosion Engineering”, Mars Fontana, McGraw-Hill, 1986. z “Corrosion and Corrosion Control”, H. H. Uhlig and R. W. Revie, John Wiley & Sons, 1985. z Web Site of Dr. R. A. (Bob) Cottis. z Web Site of Dr. Floyd Beckford 49