Acid, Base, Neutralisation, Corrosion, and Decomposition

Standard Reactions – Acid, Base, Neutralisation, Corrosion, and Decomposition
(10.1 e)
Read and answer the questions below.
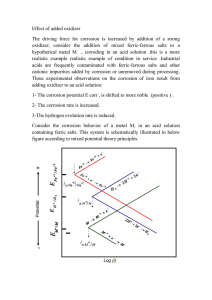
Corrosion reactions are those in which metal is “eaten away” by substances in the air or water. One example of corrosion is “dry” corrosion, which occurs when metal reacts with oxygen in the air, causing deterioration of the metal.
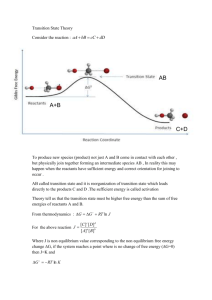
In decomposition reactions one single compound breaks down into two or more simpler chemicals. An example of this is the decomposition of zinc carbonate, represented in this equation.
ZnCO
³
(s) ZnO(s) + CO
²
(g)
Acids are substances that have a sour taste and are very corrosive. They react with solids, eating them away.
Bases taste bitter, feel slippery and may also be corrosive. Ammonia and sodium bicarbonate are examples of bases.
Neutralisation is the name given to the chemical reaction in which an acid and base react with each other to produce the neutral substance water.
Write full sentence answers
1.
What is a corrosion reaction?
2.
What is a decomposition reaction?
3.
What is an acid?
4.
What is a base?
5.
What is neutralisation?