T.C. DOKUZ EYLÜL ÜNİVERSİTESİ MÜHENDİSLİK FAKÜLTESİ
advertisement
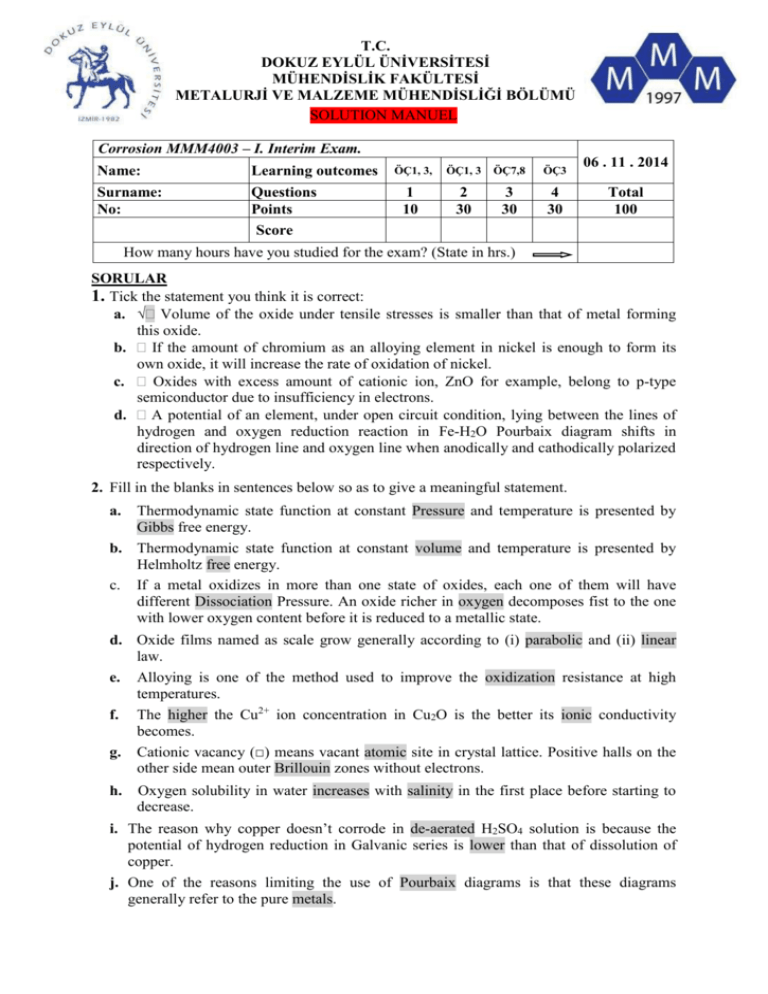
T.C. DOKUZ EYLÜL ÜNİVERSİTESİ MÜHENDİSLİK FAKÜLTESİ METALURJİ VE MALZEME MÜHENDİSLİĞİ BÖLÜMÜ SOLUTION MANUEL Corrosion MMM4003 – I. Interim Exam. Name: Learning outcomes Surname: No: Questions Points Score ÖÇ1, 3, ÖÇ1, 3 ÖÇ7,8 ÖÇ3 1 10 2 30 3 30 4 30 06 . 11 . 2014 Total 100 How many hours have you studied for the exam? (State in hrs.) SORULAR 1. Tick the statement you think it is correct: a. Volume of the oxide under tensile stresses is smaller than that of metal forming this oxide. b. If the amount of chromium as an alloying element in nickel is enough to form its own oxide, it will increase the rate of oxidation of nickel. c. Oxides with excess amount of cationic ion, ZnO for example, belong to p-type semiconductor due to insufficiency in electrons. d. A potential of an element, under open circuit condition, lying between the lines of hydrogen and oxygen reduction reaction in Fe-H2O Pourbaix diagram shifts in direction of hydrogen line and oxygen line when anodically and cathodically polarized respectively. 2. Fill in the blanks in sentences below so as to give a meaningful statement. a. Thermodynamic state function at constant Pressure and temperature is presented by Gibbs free energy. b. Thermodynamic state function at constant volume and temperature is presented by Helmholtz free energy. c. If a metal oxidizes in more than one state of oxides, each one of them will have different Dissociation Pressure. An oxide richer in oxygen decomposes fist to the one with lower oxygen content before it is reduced to a metallic state. d. Oxide films named as scale grow generally according to (i) parabolic and (ii) linear law. e. Alloying is one of the method used to improve the oxidization resistance at high temperatures. f. The higher the Cu2+ ion concentration in Cu2O is the better its ionic conductivity becomes. g. Cationic vacancy (□) means vacant atomic site in crystal lattice. Positive halls on the other side mean outer Brillouin zones without electrons. h. Oxygen solubility in water increases with salinity in the first place before starting to decrease. i. The reason why copper doesn’t corrode in de-aerated H2SO4 solution is because the potential of hydrogen reduction in Galvanic series is lower than that of dissolution of copper. j. One of the reasons limiting the use of Pourbaix diagrams is that these diagrams generally refer to the pure metals. 3. a. Describe how polarization resistance, E I , can be measured by Tafel polarization and drawing the linear relationship between E and i. A.3a When a metal electrode is polarized within a potential range of 40 mV around Ecor with a constant scanning rate starting at a potential 10 mV below and going 10 mV above Ecor (for exp. -300 mV), change in electrode potential is recorded as a function of current density, E-i. as shown in Fig 3. The slope of this curve around zero current gives the polarization resistance E I . b. When a potential ± 10 mV above or below corrosion potential was externally applied on a piece of iron exposed to a corrosive environment, a current density of 0,9x10-5 Amp/cm2 was measured to flow through a corrosion cell. Anodic and cathodic Tafel constants were measured as 0,06 and 0,07 Volt respectively. Calculate corrosion current density according to the polarization resistance formula, ∆E/∆I, developed by Stern. A.3b Making use of Stern equation 1111,11 0 ,0042 2 ,3 xikor x( 0 ,13 ) bA .bK E I 2,3 ikor (bA bK ) ikor ; 0,01 0,06 x0,07 5 0,9.10 2,3 ikor( 0,06 0,07 ) 0,0042 0,0042 1,37x10-5 Amp/cm 2 1111,11x 2,3x0,13 306,66 4. Investigate thoroughly whether a piece of zinc plate would corrode in a de-aerated 0,01M ZnCl2 solution with pH=2 by considering the reactions and data provided. Zn Zn2+ + 2eE o (Zn / Zn2 ) 0,763 Volt 2H+ + 2e- H2 (gas) E o ( H / H 2 ) 0,0 Volt Assume an activity correction constant in 0,01M ZnCl2 solution, = 0,67 and a hydrogen activity in the reaction of 1 atm. A4. 1. Zn Zn2+ + 2e+ 2. 2H+ + 2e- H2 (gaz) 1 + 2 = Zn+2H+ Zn+2 + H2 E o ( Zn / Zn 2 ) 0,763Volt E o ( H / H 2 ) 0,0Volt Standard electrode potential, Eo= 0,763 Volt Electrode p otential of the total reaction according to Nernst equation under the given condition: 𝐸 = 𝐸 𝑜 − 0,0592 2 𝑙𝑜𝑔 𝑎𝑍𝑛+2 𝑥 𝑃𝐻2 [𝐻 + ]2 = 0,763 − 0,0592 2 𝑙𝑜𝑔 0,67𝑥10_2 𝑥 1 [𝐻 + ]2 = 0,709 Volt. Here one must remember that 𝑎𝑍𝑛+2 = . [𝐶] and –log[H+] = pH. Since E is positive, change in free energy according to equation, ∆𝐺 = −𝑧𝐹E, ∆𝐺0 Therefore reaction will proceed in the direction indicated and zinc will corrode accordingly. Exam duration: 70 min A. ÇAKIR: wish you success