CHEM 261 Practice Exam: Lewis Structures, Resonance, Hybridization
advertisement

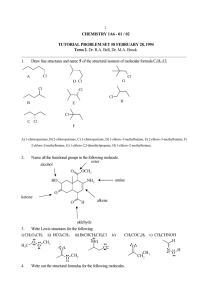
CHEM 261: FIRST TERM EXAMINATION – PRACTICE DISCLAIMER: THESE QUESTIONS ARE REPRESENTATIVE OF WHAT WILL BE ON THE MIDTERM, BUT ARE NOT NECESSARILY THE SAME AS WHAT WILL BE ON THE MIDTERM! (MORE QUESTIONS THAN WILL BE ON THE MIDTERM) LEWIS STRUCTURES Draw a Lewis structure for each of the following compounds. Include all nonbonding (lone) pairs of electrons. Draw a bond line formula for each carbon-containing compound. a. HOCN b. N2H4 c. CH2CHCO2H d. CH3CH2SO3¯ e. CH2CHCHNCH3 f. NCCH2COCH2CHO g. CH3N2+ h. CH3OSO2OCH3 i. (CH3)4NCl FORMAL CHARGE a. Assign all missing formal charges to the molecules below. Indicate the overall (net) charge on the molecule. N O O CH3 C CH3 O CH3 O CH3 B CH3 CH3 NH3 b. For each of the following structures, draw in all missing lone pairs of electrons. H N O O O RESONANCE, CURVED ARROWS a. Draw all remaining significant resonance forms for the following. Include curved arrows. Indicate and explain which is the major resonance form(s) and draw the resonance hybrid. O O O O N O N N N N O O O b. For each of the molecules below, draw the resonance form that you get from pushing the curved arrows shown. O N O OH c. For each pair of resonance forms below, draw the curved arrows that get you from one structure to the next. N N d. For each pair of structures, determine which is more stable (lower in energy). Use resonance to explain your answers. or or or O N or HYBRIDISATION, STRUCTURAL FORMULAS a. Ignoring resonance effects, answer the following questions about amoxicillin, an antibiotic from the penicillin family. H2N O O O HN N OH H HO S amoxicillin i. Predict the hybridisation of every atom, other than hydrogen. ii. How many -bonds does amoxicillin possess? iii. Indicate a C s-character. H bond containing a carbon atom having an orbital with 33% b. Ignoring resonance effects, identify the hybridisation state of each atom in the compounds below (exception: hydrogen). Determine the molecular formula for each compound. O O O O N ISOMERISM/FUNCTIONAL GROUPS a. Which of the following compounds show cis-trans isomerism? Where appropriate, draw the cis and trans isomers. CH3CH CHCH3 CH3 CH3CH C C C CH3 CH2CH3 CH2CH2CH3 CH2 FClC C(CH3)2 CClF b. Give the relationships between the following pairs of structures. The possible relationships are: same compound, resonance forms of the same compound, cis-trans isomers, constitutional isomers, not isomers (different molecular formula). CH3 CH3 and CH3 and CH3 F and CH2FCH2CH2CH2F and F O CH3OCH2CH3 and H Cl Cl F and F H H H and O F H F H O NH2 and Cl Cl H H c. Draw structures for: i. Two constitutional isomers of formula C2H7N ii. Two constitutional isomers of formula C3H8O iii. Two stereoisomers of molecular formula C2Cl2F2 iv. All constitutional isomers of C6H14 (5 in total) v. All constitutional isomers of C4H9Br (4 in total) vi. Six constitutional isomers of molecular formula C8H14 NH2