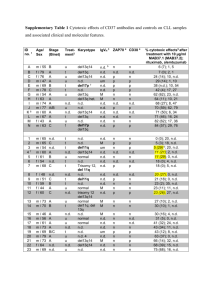
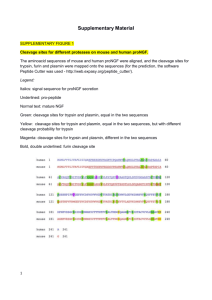
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
PHAGOCYTES
Quiescent and activated mouse granulocytes do not express granzyme A and B or
perforin: similarities or differences with human polymorphonuclear leukocytes?
Praxedis Martin, Reinhard Wallich, Julian Pardo, Arno Müllbacher, Markus Munder, Manuel Modolell, and Markus M. Simon
Polymorphonuclear leukocytes have been
shown to use a multitude of effector functions to combat pathogens and tumors,
including enzymes, defensins, and toxic
products such as oxygen radicals and
nitrogen oxides. Recent studies provided
evidence for the expression of granzymes (gzms) and perforin (perf) within
the cytotoxic arsenal of human neutrophils, the validity of which was questioned by 2 subsequent studies. We have
now used cytology, intracellular flow cy-
tometry, enzymatic assays, immunoelectron microscopy, and quantitative reverse
transcriptase–polymerase chain reaction
to obtain evidence of the presence of
gzms and/or perf in mouse Gr-1ⴙ granulocyte populations. The data obtained
clearly demonstrate that neither in vitro–
nor in vivo–derived mouse granulocytes
synthesize gzmA and gzmB or perf, even
following infection/immunization with
pathogens or pathogen-derived material.
A parallel comparable analysis on the
expression of gzmB in human neutrophils from 3 healthy control subjects and
4 patients with diverse diseases failed to
detect gzmB expression. The data indicate that polymorphonuclear leukocytes
from mice and humans lack the 3 cytotoxic effector molecules, gzmA, gzmB,
and perf, generally associated with natural killer and cytotoxic T lymphocytes.
(Blood. 2005;106:2871-2878)
© 2005 by The American Society of Hematology
Introduction
Perforin (perf) and granzymes (gzms) are major components of
cytoplasmic granules from natural killer (NK) cells and cytotoxic T
lymphocytes (CTLs) and are critical cytolytic effector molecules in
NK/CTL-mediated apoptosis and are required for the control of
intracellular pathogens and tumors.1-10 In mice and humans, gzms,
in particular gzmA and gzmB, and perf are produced mainly by the
majority of NK cells and CTLs, a fraction of CD4⫹ T cells,11-13 and
to a lesser extent by related cell types, such as metrial gland cells,14
but not by polymorphonuclear leukocytes (granulocytes/neutrophils) and monocytes.15-17
Two recent studies provided evidence that gzms and perf are also
produced by human polymorphonuclear neutrophils (PMNs).18,19 These
reports were intriguing in light of the known biologic effects of gzms
and perf in NK/CTL-mediated immune responses8,10,20,21 and the role of
PMNs as a first-line defense against microbial pathogens22,23 and
tumors.24,25 However, the assumption that PMNs use the same set of
molecules as NK/CTL to combat pathogens and tumors was challenged
by 2 subsequent studies.26,27 To date, the discrepancy of the 4 studies,
which used similar techniques for the enrichment of PMNs and to
analyze the expression of GZM- and PRF1-specific transcripts and/or
proteins, has not been resolved.
We have now analyzed in detail the expression of gzmA,
gzmB, and perf in in vitro–generated and ex vivo–derived
mouse granulocytes by combining cytology, correlative cell
surface phenotypic analysis, intracellular flow cytometry, enzyme assays, immunoelectron microscopy (IEM), and reverse
transcriptase–polymerase chain reaction (RT-PCR) analysis. In
addition we have investigated the expression of gzmB in human
PMNs from healthy individuals and a panel of patients with
distinct diseases.
From the Max-Planck-Institut für Immunbiologie, Metschnikoff Laboratory, Freiburg,
Germany; the Institut für Immunologie, Universitätsklinikum Heidelberg, Heidelberg,
Germany; the Medizinische Klinik 5, Universität Heidelberg, Heidelberg, Germany;
and the John Curtin School of Medical Research, Australian National University,
Canberra, Australia.
(7000188979) (J.P.).
Materials and methods
Mouse strains and cell lines
Inbred C57BL/6 (B6) and mouse strains deficient for gzmA (gzmA⫺/⫺),
gzmB (gzmB⫺/⫺), and gzmA ⫻ B (gzmA ⫻ B⫺/⫺) bred on the B6 background were maintained at the Max-Planck-Institut für Immunbiologie,
Freiburg, Germany, and analyzed for their genotypes as described.4,5 Male
mice of 8 to 12 weeks of age were used in all experiments and were
conducted in accordance with the ethical guidelines of the Federation of
European Laboratory Animal Science Association.
The mouse cell lines 1.3E6SN (CTL; perf⫹, gzmA⫹, gzmB⫹28) and
EL4.F15 (thymoma; perf⫺, gzmA⫺, gzmB⫺5) were used as control cells for
the analysis of perf- and gzm-specific transcripts and proteins.
Cell lines were cultured in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (FCS) and 2-mercapto
ethanol (10⫺5 M) at 7% CO2, as described29; for 1.3E6SN cells, medium
was supplemented with 10% Concanavalin A supernatant (ConA SN).
ConA SN was used as cytokine source for expansion of mouse T cells. To
produce ConA SN the SN of rat spleen cells was previously stimulated with
ConA (5 g/mL, 2 days) and subsequently treated with 20 mg/mL
␣-methyl-D-mannopyranosid to block residual ConA activity.
In vitro–propagated alloreactive T-cell lines (H-2b anti–H-2d) were
generated by weekly restimulation with irradiated spleen cells and ConA
SN, as described.30 For analysis of intracellular gzmA/gzmB expression,
alloreactive T-cell lines (⬃ 85%-90% CD8⫹) of B6, gzmA⫺/⫺, and
gzmB⫺/⫺ mice obtained after the third in vitro stimulation were used.
Reprints: Markus M. Simon, Max-Planck-Institut für Immunbiologie, Stübeweg
51, Freiburg, Germany; e-mail: simon@immunbio.mpg.de.
Submitted April 14, 2005; accepted June 10, 2005. Prepublished online as
Blood First Edition Paper, July 5, 2005; DOI 10.1182/blood-2005-04-1522.
The publication costs of this article were defrayed in part by page charge
payment. Therefore, and solely to indicate this fact, this article is hereby
marked ‘‘advertisement’’ in accordance with 18 U.S.C. section 1734.
Supported in part by an Alexander von Humboldt Foundation Fellowship
© 2005 by The American Society of Hematology
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
2871
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2872
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
MARTIN et al
Isolation of PBMCs and PMNs from human blood
Blood was taken from 4 healthy donors (2 men, 2 women) and 4 patients.
The patients were from the University Hospital Heidelberg, Germany.
Patient 1 was a 28-year-old woman with Still syndrome, rheumatoid
arthritis, acute inflammatory episode with fever, joint pains, and high
C-reactive protein (CRP; 150 mg/L). Patient 2 was a 70-year-old woman
with chronic lymphatic leukemia, fever, and tumor progression (CRP, 30
mg/L). Patient 3 was a 81-year-old man with B-non-Hodgkin-lymphoma
with fever (no CRP increase, no infection). Patient 4 was a 56-year-old
woman with acute progressive myeloid leukemia with fever, pneumonia,
and sepsis (CRP, 86 mg/L). Cells from one healthy donor were used for
cytospin analysis, cells of the other 3 donors were used for fluorescenceactivated cell sorting (FACS) staining.
Blood was taken by venous puncture using EDTA (ethylenediaminetetraacetic acid)–coated tubes (Sarstedt, Nürnbrecht, Germany), and cells were separated
on Ficoll Paque gradient (Amersham Pharmacia Biotech, Freiburg, Germany)
according to established protocols. The interphase containing the peripheral
blood mononuclear cells (PBMCs) was removed and stored on ice. The pellet
containing the PMNs and the erythrocytes were mixed with phosphate-buffered
saline (PBS) and 3% Dextran (Amersham Pharmacia Biotech; diluted in PBS)
was added 1:1 and mixed. After sedimentation of erythrocytes at room
temperature for 20 minutes the supernatant (SN) was removed, cells were
resuspended in buffer containing 155 mM NH4Cl/10 mM KH2CO3/0.1 mM
EDTA in H2O, and incubated for 15 minutes on ice to lyse resting erythrocytes.
After centrifugation the SN was discarded, and the pellet containing neutrophils
was resuspended in RPMI.
Approval was obtained from the Medizinische Klinki 5, University
Heidelberg institutional review board for these studies. Informed consent
was provided according to the Declaration of Helsinki.
In vitro generation of mouse granulocytes
Mouse granulocyte populations were generated from bone marrow (BM). BM
cells were rinsed from femur and tibia (needle 23G; Terumo, Roma, Italy) with
MEM/1 ⫻ nonessential amino acids/1 mM sodium pyruvate/1 mM L-glutamine/
10⫺5 M 2-mercapto ethanol (MEM-complete [MEM-comp]), adjusted to 1 ⫻ 106
cells/mL, and cultivated in a 24-well plate (MEM-comp ⫹ 20% horse serum
[HS]; Cell Concepts, Umkirch, Germany; 1 mL/well; 37°C, 7% CO2) in the
presence of 5 ng/mL granulocyte colony-stimulating factor (G-CSF; PeproTech,
Rocky Hill, NJ) and 5 M hydrocortisone (Sigma-Aldrich, Taufkirchen,
Germany). After 5 days, 500 L medium was replaced by 500 L fresh
MEM-comp ⫹ 20% HS for a further 4 days.
Infection of mice and activation of leukocytes and granulocytes
in vivo and in vitro
Mice were infected with 5 ⫻ 103 colony-forming units (CFUs) Listeria monocytogenes (2.5 ⫻ 104 CFU/mL; wild-type, strain EGD; PBS) or injected with 2 g
lipopolysaccharide (LPS; Salmonella abortus equi, smooth form; provided by C.
Galanos, Max-Planck-Institut für Immunbiologie, Freiburg, Germany; 10 g/mL
in PBS) intraperitoneally. At indicated time points, the peritoneal cells were
recovered by rinsing with PBS and stored on ice for further analysis.
Mice were infected with 1 ⫻ 105 plaque-forming units lymphocytic
choriomeningitis virus (LCMV)–WE intraperitoneally according to established protocols.5,31 At day 8 after infection, spleens were removed for
further analysis.
In vitro–enriched granulocytes (BM cells, day 9, G-CSF) were incubated in the presence of either LPS (Salmonella minnesota, rough form;
provided by C. Galanos; 1 g/mL) and recombinant lipoprotein outer
surface protein A (Lip-OspA; 10 g/mL; GlaxoSmithKline Beecham,
Rixensart, Belgium) in MEM-comp ⫹ 20% HS for 8 hours or with 50
g/L ⫻ 105 cells zymosan A (Sigma-Aldrich) in MEM-comp ⫹ 20% HS
for 90 minutes.
Mouse splenocytes were activated with 5 g/mL ConA (Amersham
Pharmacia Biotech) with or without 10% IL-2 (ConA SN) in MEM (10%
FCS/10⫺5 M 2-mercaptoethanol) for 2 to 3 days.
Morphologic analysis of cells by cytospin
Cells were washed with PBS, and 1 ⫻ 105 cells/150 L PBS were centrifuged on
microscope slides in a Cytospin 3 (Shandon Thermo Electron, Pittsburgh, PA).
The cells were incubated with 150 L May-Grünwald (Merck, Darmstadt,
Germany) for 2 minutes, the same volume of H2O was added for a further 3
minutes. After washing the slides twice with H2O, cells were stained with Giemsa
(diluted 1:20 in H2O; Sigma-Aldrich) for 20 minutes, washed, and embedded
with Entellan (Merck). The images were taken using a Zeiss Axioskop 10
microscope, a Zeiss Axiocam as analysis camera, and Zeiss Vision 3.0 as
software (Carl Zeiss, Jena, Germany). The objective used was a Zeiss
PlanNeofluor, with a magnification of 40 ⫻ and a numeric aperture of 0.75.
Flow cytometry
All monoclonal antibodies (mAbs) for surface staining, except anti–(␣) CD4
mAb, ␣CD8 mAb (both from Coulter, Marseille, France; used for the healthy
individuals 2 and 3), and ␣CD66b mAb (Coulter, Marseille, France) were
obtained from BD Pharmingen, Heidelberg, Germany. Splenocytes, mouse
granulocytes, and human PBMCs and PMNs were washed with FACS-washing
buffer (PBS, 5% FCS, 0.1% NaN3) as previously described31 and incubated with
10 L mAbs for 20 minutes. ␣Mouse (m) mAb used were fluorescein
isothiocyanate (FITC)– and phycoerythrin (PE)–labeled ␣B220 (clone RA36B2), PE-labeled ␣CD8 (clone 53-6.7), FITC- and PE-labeled ␣Gr-1 (clone
RB6-8C5), FITC-labeled ␣Mac-1 (clone M1/70), FITC- and PE-labeled ␣NK1.1
(clone PK136), and FITC- and PE-labeled ␣Thy1.2 (clone 53-2.1). All fluorescence-conjugated ␣m mAbs were diluted in an ␣Fc receptor antibody (clone
2.4G2), 1:50 for FITC-labeled mAb and 1:100 for PE-labeled mAb. ␣Human
(hu) mAbs used were FITC-labeled ␣CD4 (clone RPA-T4), PE-labeled ␣CD8
(clone RPA-T8), and FITC-labeled ␣CD66b (clone 80H3; Coulter) for all
patients and the healthy individual 1; FITC-labeled ␣CD4 (Coulter), PE-labeled
␣CD8 (Coulter), and FITC-labeled ␣CD66b (clone G10F5) for the healthy
individuals 2 and 3. The ␣hu mAbs were diluted in washing buffer. Stained cells
were washed twice and fixed in 100 L PBS containing 1% paraformaldehyde
(PFA), examined in a FACSCalibur (Becton Dickinson), and analyzed with
CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
For analysis of intracellular gzmA and gzmB, 1.3E6SN, EL4.F15,
splenocytes from LCMV-immune mice (day 8 after infection), mouse
granulocytes, and human PBMCs or PMNs were stained with surface
markers as described in “Flow cytometry” and then fixed with 100 L PBS
containing 2.5% PFA for 15 minutes at 4°C. Subsequently, cells were
incubated in 100 L permeabilizing buffer (PBS, 5% FCS, 0.1% NaN3,
0,1% Saponin; Roth, Karlsruhe, Germany) for 10 minutes at 4°C, then
stained for 45 minutes at 4°C with 40 L rabbit ␣gzmA immune serum (IS)
(diluted in permeabilizing buffer) or with allophycocyanin (APC)–labeled
mouse ␣gzmB mAb (clone GB12; Caltag, Burlingame, CA; diluted in
permeabilizing buffer), and subsequently washed with permeabilizing
buffer; in the case of gzmA, cells were stained with 40 L FITC-labeled IS
goat ␣rabbit immunoglobulin G (IgG) as secondary Ab (Jackson Dianova,
West Grove, PA; diluted in permeabilizing buffer; 1:500). As isotype
controls, rabbit IgG (Jackson Dianova) for gzmA and APC-labeled mouse
IgG (BD Pharmingen) for gzmB were used. Stained cells were washed
twice in permeabilizing buffer, fixed in 100 L PBS containing 1% PFA,
examined in a FACSCalibur, and analyzed with CellQuest software.
For cell sorting, the enriched granulocytes (BM, day 9, G-CSF) were
washed with FACS-washing buffer and incubated with 50 to 100 L
FITC/PE-labeled ␣Gr-1 mAb (diluted 1:50/1:100 in ␣Fc receptor SN [clone
2.4G2]) 20 minutes at 4°C. Cells were washed in PBS, homogenized with a
separation filter (Miltenyi Biotec, Bergisch Gladbach, Germany), and
positively sorted with the cell sorter MoFlo (Cytomation, Freiburg, Germany).
Enzymatic assays
Cell lysates from 1.3E6SN, EL4.F15, positively sorted (␣CD8 Microbeads;
Milteny, Biotec; according to manufacturers instruction) CD8⫹ LCMVimmune splenocytes (day 8 after infection) and from in vitro–enriched
granulocytes (BM, day 9, G-CSF) were prepared in 0.1% Triton X-100/10
mM Tris [tris(hydroxymethyl)aminomethane]–HCl in H2O, pH 7.5 (1 hour
on ice) and tested as described30 on the following substrates: Ac-Ile-Glu-ProAsp-pNA (gzmB; Bachem, Weil am Rhein, Germany), Suc-Phe-Pro-PhepNA (CathepsinG; Bachem). The absorbance was examined in a spectrophotometer SpectraMax 190 (Molecular Devices, Ismaning/Munich, Germany;
wavelength 405 nm/690 nm) and analyzed with SOFTMax Pro software
(Molecular Devices).
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
MOUSE GRANULOCYTES LACK GRANZYME A/B AND PERFORIN
Electron microscopy
TAG CTG CAC-3⬘) amplifying a 272-bp segment. The primers for Hprt1,
Gzma, Gzmb, and Prf1 were synthesized by Hermann (Freiburg, Germany), for
Ctsg by Thermo Electron (Ulm, Germany). The PCR products were analyzed by
gel electrophoresis (2% agarose), stained with ethidium bromide, and analyzed
using an UV-transluminator (Vilber Lourmat, New Ark, NJ). As size markers,
1-kilobase (kb) DNA marker was used (BioLabs, Frankfurt/Main, Germany).
In vitro–enriched granulocytes and 1.3E6SN cells were fixed in 4% PFA in
0.2 M PHEM buffer (240 mM PIPES [Piperazine-1,4-bis(2-ethanesulfonic
acid)]/100 mM HEPES [N-2-hydroxyethylpiperazine-N⬘-2-ethanesulfonic
acid]/40 mM EGTA [ethyleneglycoltetraacetic acid]/8 mM MgCl2, pH 6.),
stored in 0.5% PFA in 0.1 M PHEM buffer, and then processed for ultrathin
cryosectioning as previously described.32 Cryosections (45 nm) cut at
125°C using diamond knives (Drukker, Cuijk, The Netherlands) in an
ultracryomicrotome (Leica Aktiengesellschaft, Vienna, Austria) were transferred with a mixture of sucrose and methylcellulose onto formvar-coated
copper grids.33 The grids were placed on 35-mm Petri dishes containing 2%
gelatin. For double immunolabeling, a published procedure34 was followed
with 10- and 15-nm protein-A–conjugated colloidal gold probes, using
␣mgzmA mAb.30 After immunolabeling, the cryosections were embedded
in a mixture of methylcellulose and uranyl acetate and examined with a
Philips CM 10 electron microscope (Philips, Eindhoven, The Netherlands).
Images were captured on negatives, and prints were scanned and processed
with Adobe Photoshop (Adobe, San Jose, CA). Original magnification,
12 500 ⫻.
2873
Results
Phenotype of BM-derived and in vitro–propagated
mouse granulocytes
Granulocytes were generated from mouse BM by in vitro cultivation
with G-CSF (day 9) and further enriched by FACS sorting with mAb to
Western blot analysis
Intracellular gzmA was determined in cell lysates from magnetic-activated
cell sorting (MACS)–sorted LCMV-immune CD8⫹ cells of B6, gzmA⫺/⫺,
and gzmB⫺/⫺ mice by Western blotting under nonreducing conditions. The
␣mgzmA mAb (clone 7.1) was generated as decribed,30 the ␣m-actin
mAb (clone C-11) was obtained from Santa Cruz Biotechnology (Santa
Cruz, CA). Then the blot was stained with peroxidase-conjugated secondary antibodies, goat ␣rat or rabbit ␣goat, respectively, from Jackson
Dianova. Subsequently, a peroxidase substrate for enhanced chemiluminescence (ECL) from Pierce (Rockford, IL) was used for detection. Purified
gzmA protein (50 g/mL)35 was used as positive control.
Probing for mRNA transcription
Total RNA was extracted from up to 5 ⫻ 106 cells, using the QIAshredder spin
columns, the RNeasy Mini Kit, and the RNase-free DNase Kit (all from Qiagen,
Hilden, Germany) according to the manufacturer’s instructions. mRNA was
transcribed by incubating total RNA with random hexamer primer (660 ng/L;
Pharmacia, Freiburg, Germany), RNasin inhibitor (20 U; Promega, Madison,
WI), deoxyribonucleoside triphosphate (0.5 mM; Qbiogene, Heidelberg, Germany), and Omniscript reverse transcriptase (4 U; Qiagen) as advised by the
manufacturer. Reverse transcription was performed in a Thermocycler PTC-200
DNA-Engine (MJ Research, Waltham, MA). The transcription reaction profile
was as follows: 37°C for 60 minutes and 70°C for 10 minutes. The resulting
cDNA was used as a template for RT-PCR hypoxanthin:guanine phosphoribosyltransferase (Hprt1), Prf1, Gzma, and Gzmb amplification. Polymerase chain
reaction (PCR) for Hprt1, Prf1, Gzma, and Gzmb was carried out in a
Thermocycler PTC-200 as described in “Probing for mRNA transcription.” The
PCR reaction profile for Hprt1, Prf1, Gzma, and Gzmb was as follows: 1 cycle at
94°C for 2.5 minutes as an initial denaturation step; then denaturation at 94°C for
20 seconds; annealing at 56°C for 20 seconds for Hprt1 or 55°C for 20 seconds
for Prf1, Gzma, and Gzmb; extension at 72°C for 20 seconds for Hprt1 or 72°C for
30 seconds for Prf1, Gzma, and Gzmb (35 cycles); followed by further incubation
for 3 minutes at 72°C (1 cycle). The PCR reaction profile for the cathepsin G gene
(Ctsg) was as follows: 1 cycle at 95°C for 1 minute as an initial denaturation step,
then denaturation at 94°C for 30 seconds, annealing at 48°C for 30 seconds,
extension at 70°C for 1 minute (35 cycles), followed by further incubation for 8
minutes at 70°C. The following primers were used for amplification: Hprt1specific primers, sense (5⬘-GCT GGT GAAAAG GAC CTC T-3⬘) and antisense
(5⬘-CAC AGG ACT AGA ACA CCT GC-3⬘ amplifying a 249-base pair (bp)
segment; Prf1-specific primers, sense (5⬘-GAG CCC CTG CAC ACATTA CTG
GAA-3⬘) and antisense (5⬘-ACA TTC TCA AAG TCC ATC T-3⬘) amplifying a
380-bp segment; Gzma-specific primers, sense (5⬘-GGG GAT CTA CAA CTT
GTA CGG-3⬘) and antisense (5⬘-ATT GCA GGA GTC CTT TCC ACC AC-3⬘)
amplifying a 291-bp segment; Gzmb-specific primers sense (5⬘-TCA GGC TGC
TGA TCC TTG ATC G-3⬘) and antisense (5⬘-ATG AAG ATC CTC CTG CTA
CTG C-3⬘) amplifying a 135-bp segment; Ctsg-specific primers, sense (5⬘-CAT
CCA AAT GCG AGA AAG GAC-3⬘) and antisense (5⬘-CAT CTG CAC TCT
Figure 1. Phenotypic analysis of in vitro–generated and positively selected granulocytes from B6 BM cells. (A) B6 granulocytes were generated from B6 BM cells by
cultivation with G-CSF for 9 days. Enriched granulocytes were further enriched by positive
selection via FACS using ␣mGr-1 mAb. All 3 populations were stained with mAb specific for
Gr-1, NK1.1, B220, Thy1.2, and Mac-1 and analyzed with the FACSCalibur. SSC indicates
side scatter. (B) Freshly isolated B6 BM cells and in vitro–propagated and FACS-sorted
(␣mGr-1 mAb) granulocytes were centrifuged on microscope slides and stained with
May-Grünwald/Giemsa as described in “Materials and methods.” Arrows indicate polymorphonuclear cells; arrowheads, mononuclear cells. (C) Not activated B6 spleen cells and B6
spleen cells cultivated in vitro in the presence of either ConA (5 g/mL) or ConA/ConA SN
(5 g/mL; 10% final) were subjected to FACS analysis (FACSCalibur), using mAb to Gr-1,
NK1.1, B220, and/or Thy1.2. FSC indicates forward scatter. Numbers in graphs indicate
the percentages of gated cells.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2874
MARTIN et al
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
Figure 2. Mouse Gr-1ⴙ granulocytes do not express gzmA or gzmB intracellularly. All indicated cell populations were stained, intracellularly, with either rabbit ␣mgzmA IS followed by
FITC-labeled ␣rabbit IgG (1:500) or with APC-labeled ␣hugzmB mAb; rabbit IgG and mouse IgG were used as isotype control, as described in “Flow cytometry.” Analysis was performed
using FACSCalibur. (A) 1.3E6SN, EL4.F15:␣mgzmA IS (1:1000), ␣hugzmB mAb (1:100). (Bi) Ex vivo–derived LCMV-immune (day 8 after infection) spleen cells from B6, gzmA⫺/⫺, and
gzmB⫺/⫺ mice were stained with ␣CD8 mAb (1:100) and, subsequently, with either ␣mgzmA IS (1:100), or ␣hugzmB (1:1000) mAb for intracellular expression of gzms and analyzed as
described in “Flow cytometry.” (Bii) In vitro–propagated alloreactive (H-2b anti–H-2d; third stimulation) T cells from B6, gzmA⫺/⫺, and gzmB⫺/⫺ mice were treated as in panel Bi. (Ci) In
vitro–generated granulocytes (BM cells, day 9, G-CSF) from either B6, gzmA⫺/⫺, or gzmB⫺/⫺ mice were stained with the ␣Gr-1 mAb and, subsequently, with either ␣mgzmA IS (1:100) or
␣hugzmB (1:10) mAb for intracellular expression of gzms, as described in “Flow cytometry.” (Cii) Alternatively, in vitro–enriched B6 granulocytes were sensitized to either LPS (1 g/mL) or
Lip-OspA (10 g/mL) for 8 hours, prior to phenotypic analysis, using ␣Gr-1 mAb and either ␣mgzmA IS (1:100) or ␣hugzmb (1:10) mAb for intracellular expression of gzms as described
above. Numbers in graphs indicate the percentages of gated cells.
Gr-1. This protocol leads to a highly purified granulocyte population
(Figure 1A): freshly isolated BM cells consisted of approximately 50%
Gr-1⫹, 3% NK1.1⫹, 44% B220⫹, 6% Thy1.2⫹, and 48% Mac-1⫹ cells
(Figure 1Ai), with approximately 94% of Gr-1⫹ cells staining in
addition for Mac-1 and approximately 2% to 3% for NK1.1, B220, or
Thy1.2. The cell population propagated in G-CSF consisted of greater
than 93% Gr-1⫹Mac-1⫹ (Figure 1Aii) and on further enrichment by
FACS of greater than 99% Gr-1⫹Mac-1⫹ with only a minority (⬃ 1%)
also staining for NK1.1, B220, or Thy1.2 (Figure 1Aiii). Cytospin
analysis revealed that the majority of Gr-1–sorted cells are granulocytes
(Figure 1B, BM-derived versus in vitro–propagated and FACS-sorted
cells). The majority of granulocytes generated under these conditions are
Gr-1⫹Mac-1⫹, with only few, if any, expressing markers for NK, B, and
T cells. However, the concern that the Gr-1 marker is not restricted to
mouse granulocytes36,37 (and information provided by manufacturer) is
supported by phenotypic data obtained with ex vivo–derived or in vitro
mitogen-stimulated spleen cells. From the approximately 7% of Gr-1⫹
cells detected in ex vivo–derived spleen cells (day 8 after infection),
approximately 16% to 20% also stained for NK1.1 or B220 and greater
than 75% for Thy1.2 (Figure 1C). Furthermore, spleen cell populations
sensitized with ConA in vitro to enrich for activated T cells contained
approximately 15% Gr-1⫹ cells, of which approximately 2%, approximately 35%, and approximately 60% also stained for NK1.1, B220, and
Thy1.2, respectively. When the same cell population was cultured in the
presence of ConA plus ConA SN, supporting polyclonal T-cell expansion, from the approximately 32% Gr-1⫹ spleen cells recovered,
approximately 17% and approximately 90% also stained for B220 and
Figure 3. In vitro–propagated and zymosan-activated B6 granulocytes do not
express gzmA or gzmB. In vitro–generated B6 granulocytes were incubated with
zymosan (50 g/L ⫻ 105 cells; 90 minutes) and subsequently analyzed following
staining with ␣Gr-1 mAb and either ␣mgzmA IS (1:100) followed by FITC-labeled
␣rabbit IgG (1:500) or ␣hugzmb mAb (1:10) as described in “Flow cytometry”; rabbit
IgG and mouse IgG were used as isotype control, as described in “Materials and
methods.” Numbers in graphs indicate the percentages of gated cells.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
MOUSE GRANULOCYTES LACK GRANZYME A/B AND PERFORIN
Thy1.2, respectively. These observations are critical for a meaningful
evaluation of data regarding expression of gzms and perf in mouse
granulocytes and underscore the necessity for a correlative phenotypic
analysis of the Gr-1⫹ cells, in particular for NK and T-cell–associated
cell surface markers, so as to exclude gzm/perf-expressing contaminating cells.
higher levels of both gzms, it is clearly shown that both, ␣mgzmA
IS or ␣hugzmB mAb, are specific and differentially stain the
respective cell populations from B6, gzmA⫺/⫺, and gzmB⫺/⫺ mice
(Figure 2Bii). Most importantly, none of the in vitro–enriched
granulocyte populations from B6, gzmA⫺/⫺, and gzmB⫺/⫺ mice
stained with ␣mgzmA IS and/or ␣hugzmB mAb (Figure 2Ci), even
after earlier activation with either LPS or the lipoprotein Lip-OspA
(Figure 2Cii; only shown for B6 granulocytes). Similar results were
obtained with in vitro–enriched B6 granulocytes previously stimulated (90 minutes) with zymosan (Figure 3). The combined data
indicate that in vitro–generated mouse granulocytes do not express
gzmA and gzmB, even after activation and expansion.
The data obtained by FACS analysis were verified by IEM
(Figure 4, only shown for gzmA; ␣mgzmA mAb; 7.1). Only
specimens of 1.3E6SN but not EL4.F15 (data not shown) were
heavily stained with ␣mgzmA mAb (7.1) in particular in cytoplasmic granules. In contrast, no or only few grains were seen in
sections of in vitro–enriched mouse neutrophils or eosinophils. The
specificity of the ␣mgzmA mAb (7.1) has been described before30
and was verified again by WB analysis, using cell lysates from
MACS-sorted CD8⫹ LCMV-immune cells from B6, gzmA⫺/⫺, and
gzmB⫺/⫺ mice as well as purified gzmA (Figure 4B).
Cell lysates from in vitro–enriched mouse granulocytes as well
as from 1.3E6SN and EL4.F15 cell lines were tested for proteolytic
activities of gzmB and cathepsin G, a granulocyte-specific protease,39 by using appropriate chromogenic substrates. 1.3E6SN
lysates contained gzmB- (19 U/106 cells) but not cathepsin
In vitro–derived mouse granulocytes do not express gzmA or
gzmB intracellularly
In vitro–enriched BM-derived granulocytes were analyzed by
FACS for intracellular expression of gzmA and gzmB by using
␣mgzmA IS or ␣hugzmB mAb, the latter is known to crossreact
with mouse gzmB.38 The specificity of both antibodies for the
respective gzm was verified by using the gzmA/gzmB-positive
CTL line, 1.3E6SN, and the gzmA/gzmB-negative thymoma line,
EL4.F15, as well as ex vivo–derived LCMV-immune spleen cells
(day 8 after infection) from B6, gzmA⫺/⫺, and gzmB⫺/⫺ mice. As
expected, only 1.3E6SN, but not EL4.F15, cells stained with
␣mgzmA IS and ␣hugzmB mAb (Figure 2A). In addition, ␣mgzmA IS stained a fraction of CD8⫹ T cells from LCMV-immune B6
and gzmB⫺/⫺ but not gzmA⫺/⫺ mice and, conversely, ␣hugzmB
mAb stained a fraction of CD8⫹ T cells from LCMV-immune B6
and gzmA⫺/⫺ but not gzmB⫺/⫺ mice (Figure 2Bi). The lower
intensities seen with T cells stained for gzmB compared with gzmA
may be due to the fact that a monospecific mAb rather than a
polyspecific IS was used in the former case. However, using in
vitro–propagated alloreactive T-cell lines, known to express much
2875
Figure 4. Ultrastructural localization of gzmA in 1.3E6SN but not in B6 neutrophils and eosinophils by immunogold electron microscopy. (A) Cryosections of
1.3E6SN cells or of in vitro–generated (BM cells, day 9, G-CSF) B6 neutrophils or eosinophils were fixed, immunogold-labeled with ␣mgzmA mAb (7.1; arrows) and
subsequently analyzed by electron microscopy. (B) Western blot (WB) analysis of lysates of MACS-sorted CD8⫹ LCMV-immune cells from B6, gzmA⫺/⫺, and gzmB⫺/⫺ mice
(5 ⫻ 105 cell equivalents/lane) and of purified gzmA protein (50 ng) using either ␣mgzmA mAb (7.1, 1:20) or ␣m-actin mAb (C-11, 1:1000) and peroxidase-labeled secondary
antibody (1:10 000). Molecular weight was as follows: gzmA, 60 kDa; -actin, 43 kDa.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2876
MARTIN et al
G–related activity (0 U/106 cells), whereas EL4.F15 lysates did not
harbor either of the 2 enzymatic activities (0 U/106 cells). In
addition, lysates from ex vivo–derived and enriched LCMVimmune CD8⫹ T cells from B6 (3 U/106 cells) but not gzmAxB⫺/⫺
mice (0 U/106 cells) expressed gzmB activity, although at much
lower levels (3 U versus 19 U/106 cells, as expected from previous
studies; M.M.S. unpublished data, February 2004) when compared
with the long-term cultured T-cell line, 1.3E6SN. Both ex vivo–
derived T-cell populations expressed low but significant levels of
cathepsin G or cathepsin G–like activity (1.3 U/106 cells). In
contrast, lysates from in vitro–enriched granulocytes, unstimulated
or LPS-stimulated, expressed only cathepsin G–related (17 U/106
cells), but not gzmB-related (0 U/106 cells), enzymatic activities.
Ex vivo–derived mouse granulocytes do not express gzmB
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
pathogens in vivo, do not express gzmA, gzmB, or perf. The
present data obtained by cytologic, intracellular flow cytometric,
enzymatic, IEM, and semiquantitative RT-PCR analyses, support
earlier studies, indicating that in mice the 3 cytolytic effector
molecules, gzmA, gzmB, and perf, are produced mainly by NK and
CTL,12,15,28 and to some extend also by metrial gland cells,14 but not
by other cells of the lymphoid or myeloid lineages.
Human granulocytes from patients with various disease
symptoms and healthy individuals do not express gzmB
In light of the published conflicting results on the expression of
gzms and perf in human neutrophils,18,19,26,27 we tested a panel of
PBMC and PMN populations from 4 healthy individuals (cells of 1
individual were used for cytospin, cells of the other 3 individuals
B6 mice were injected intraperitoneally with LPS or infected
intraperitoneally with L monocytogenes. Twenty hours and 48
hours after infection, respectively, cells were recovered from the
peritoneal cavity and analyzed by FACS for surface phenotype and
the expression of intracellular gzmB. Approximately 45% of cells
from LPS-challenged mice stained Gr-1⫹, although with differential intensity. Only approximately 3% to 4% of cells expressing
Gr-1 were also positive for NK1.1 and Thy1.2, and mainly in the
Gr-1low cell population. When tested for the expression of gzmB,
only few Gr-1hi but significantly more Gr-1low cells stained for
gzmB. In contrast to Gr-1⫹ cells, a much higher proportion from
Thy1.2⫹ cells (⬃ 13%, total) and NK1.1⫹ cells (⬃ 3%, total),
expressed gzmB (⬃ 10% and 20%, respectively) (Figure 5Ai). As
expected, none of the ex vivo–derived LPS-sensitized Gr-1⫹ cells
from gzmB⫺/⫺ mice expressed gzmB (Figure 5Aii).
The peritoneal cell population from L monocytogenes–infected
mice contained approximately 34% Gr-1⫹ cells, with approximately 74% and approximately 6% being also positive for NK1.1
and Thy1.2, respectively (Figure 5B). However, none of the Gr-1⫹
cells expressed gzmB, whereas approximately 4% of the NK1.1⫹
cells (⬃ 43%, total) and approximately 9% of the Thy1.2⫹ cells
(⬃ 22%, total) were positive for gzmB. Similar results were
obtained for gzmA (data not shown). Together, these data indicate
that ex vivo–derived Gr-1⫹ mouse granulocytes do not express
gzmA and gzmB, even during infection or on challenge with
pathogen-derived agents (LPS).
Mouse BM-derived granulocytes do not express transcripts
for gzmA, gzmB, and perf
In vitro–enriched and FACS-sorted Gr-1⫹ BM-derived granulocyte
populations from B6 and gzmB⫺/⫺ mice were assayed by RT-PCR for
the expression of Gzma, Gzmb, and Prf1-specific transcripts. None of
the 2 cell populations expressed Gzma, Gzmb, or Prf1-specific mRNA,
irrespective of whether they were stimulated with LPS or left untreated
(Figure 6A). As expected, mRNA from 1.3E6SN, but not EL4.F15, cells
expressed transcripts for Gzma, Gzmb, and Prf1. In contrast, when in
vitro–enriched but unsorted granulocyte populations were used, Gzmbspecific transcripts were detectable, the level of which was enhanced on
pretreatment with LPS (Figure 6B; lanes 2 and 3). No Gzm- or
Prf1-specific transcripts were seen in in vitro–generated and FACSenriched Gr-1⫹ cells (Figure 6B; lane 1). In contrast, specific bands for
the granulocyte-specific Ctsg were only detected in samples of granulocytes, but not in 1.3E6SN and EL4.F15 (Figure 6B). Similar results
were obtained when mRNA preparations were subjected to quantitative
RT-PCR using the light cycler system (data not shown).
In summary, these results clearly demonstrate that mouse
granulocytes, even after in vitro activation or on encounter with
Figure 5. Granulocytes from B6 mice previously challenged with LPS or L
monocytogenes in vivo do not express gzmB. Peritoneal exudate cells were
derived from B6 and gzmB⫺/⫺ mice, either previously injected with LPS (2 g;
intraperitoneally), at 20 hours after infection (A) or previously infected intraperitoneally with L monocytogenes (5 ⫻ 103 CFU), at 48 hours after infection (B), and
analyzed (FACSCalibur) for the expression of Gr-1, NK1.1, Thy1.2, and Mac-1 and
intracellularly for gzmB (␣hugzmB mAb, 1:10), as described in “Materials and
methods”; mouse IgG was used as isotype control, as described in “Materials and
methods.” Numbers in graphs indicate the percentages of gated cells.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
MOUSE GRANULOCYTES LACK GRANZYME A/B AND PERFORIN
2877
cytes are Gr-1⫹, but that they may differentially express NK- and
T-cell–specific markers, depending on their activation state. However, fractions of ex vivo–derived spleen cells or spleen cells,
previously activated in vitro with ConA or ConA plus ConA SN
were double positive for either NK1.1 and Gr-1 or Thy1.2 and Gr-1
(Figure 1C).
Phenotype analysis of human PBMCs and PMNs led to similar
observations. Thus, highly enriched PBMC populations contained
small fractions of CD56⫹ or CD3⫹ cells, which also expressed
CD66b and, vice versa, highly enriched PMNs (⬎ 95% CD66b)
contained a small fraction of cells, which expressed either CD56 or
Figure 6. In vitro–generated and FACS-sorted Gr-1ⴙ mouse granulocytes do
not express transcripts for Gzma, Gzmb, and Prf1. (A) mRNA was isolated from in
vitro–generated and FACS-enriched (Gr-1⫹) unstimulated or LPS-sensitized (1 g/
mL; 8 hours) B6 and gzmB⫺/⫺ granulocytes. Gzma, Gzmb, Prf1, or Hprt1 transcripts
were analyzed with specific primer pairs for Gzma, Gzmb, Prf1, and Hprt1 by
RT-PCR. As positive and negative controls, 1.3E6SN (lanes 6,8) and EL4.F15 (lanes
7,9) were used. Size of amplified fragments was as follows: Gzma, 291 bp; Gzmb,
135 bp; Prf1, 380 bp; and Hprt1, 249 bp. (B) mRNA was isolated from in
vitro–generated unstimulated or LPS-sensitized (1 g/mL; 8 hours) (lanes 2 and 6,7,
respectively) or in addition FACS-enriched (Gr-1⫹; lanes 1 and 5) B6, and gzmA⫺/⫺
granulocytes and analyzed as described in “Probing for mRNA transcription,” with
specific primer pairs for Gzma, Gzmb, Prf1, Ctsg, and Hprt1 by RT-PCR. Size of
amplified Ctsg fragments was as follows: 272 bp; as positive and negative controls,
1.3E6SN (lanes 8, 10), and EL4.F15 (lanes 9, 11) were used.
were stained intracellularly) and 4 patients with either an autoimmune disorder or B-cell chronic lymphatic leukemia, B-cell
non-Hodgkin lymphoma, or acute myelogenous leukemia. PBMCs
and PMNs were separated by standard methods and subsequently
applied to cytospin and FACS analysis. Both cell populations were
stained for surface markers CD4, CD8, and CD66b as well as
intracellularly for gzmB using ␣hugzmB mAb (GB12). PBMCs
from the healthy individual consisted of approximately 44% CD4⫹
and approximately 20% CD8⫹ T cells, whereas PBMCs from the 4
patients had CD4⫹/CD8⫹ T-cell ratios, ranging from approximately
74%:6% (patient 4) to approximately 30%:30% (patient 3), respectively (Figure 7A). PBNs from all individuals consisted of greater
than 98% CD66b⫹ cells with no contaminants of CD4⫹ or CD8⫹ T
cells. The polymorphonuclear nature of CD66b-enriched cells of a
healthy individual was also revealed by cytospin analysis in an
independent experiment (Figure 7B). When tested for the intracellular expression of gzmB, a significant number of CD8⫹ PBMCs
(ranging from 16% to 71%) but also a fraction of CD4⫹ PBMCs
(ranging from 0.5% to 17%) stained positive for the enzyme. In
contrast, none of the PBN populations from the healthy individuals
and the 4 patients expressed intracellular gzmB.
Our findings that only human PBMCs, in particular fractions of
CD4⫹ and CD8⫹ T cells, but none of the enriched PMN populations from the healthy individuals and 4 patients with various
disease symptoms expressed gzmB are in line with 2 recent
studies,26,27 but challenge 2 others.18,19 Final proof whether human
PMNs are able to produce gzms and/or perf under certain
conditions is still pending. A cautionary note regarding proof of
principle needs to be added to avoid erroneous conclusions. This
pertains, in particular, to enrichment of granulocytes, via mAb: We
found that the majority of cytologically defined mouse granulo-
Figure 7. GzmB is only expressed in fractions of CD4ⴙ and CD8ⴙ PBMCs, but
not in CD66bⴙCD4ⴚCD8ⴚ PMNs of 4 patients and 3 healthy individuals.
(A) PBMCs and PMNs were isolated from 4 patients (1-4) and 3 healthy individuals
and analyzed (FACSCalibur) by staining with either mAb to CD4 and CD8 (PBMCs
and PMNs), or mAb to CD66b (PMNs), and subsequently with ␣hugzmB mAb (1:50)
as described in “Materials and methods.” Numbers in graphs indicate the percentages of gated cells. (B) Similarly prepared PBMCs and PMNs from another individual
showing a comparable phenotype as the healthy individuals mentioned in panel A
were centrifuged on microscope slides and stained with May-Grünwald/Giemsa as
described in “Materials and methods.” Arrowheads indicate mononuclear cells;
arrows, polymorphonuclear leukocytes.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2878
BLOOD, 15 OCTOBER 2005 䡠 VOLUME 106, NUMBER 8
MARTIN et al
CD3 in addition to CD66b. This also holds true when another
marker, CD15, mainly associated with human PMNs,18,26 was
applied (data not shown). Thus, granulocyte populations/PMNs
selected solely by Gr-1 (mouse) or CD66b/CD15 (humans) may
either contain granulocyte/neutrophil subpopulations expressing
NK/T-cell markers or may be contaminated with cells from other
lineages, in particular NK, NKT (natural killer T), and T cells,
which are the main producers of gzms and perf.
Discussion
The data presented here provide strong supportive evidence that mouse
granulocytes do not express gzmA, gzmB, and perf under physiologic or
pathophysiologic conditions. This is supported by the lack of Prf1- and
Gzma-/Gzmb-specific mRNA and the respective proteins in in vitro–
generated or ex vivo–derived granulocyte populations, as revealed by
cytologic, intracellular flow cytometry, enzymatic, IEM, and RT-PCR
analyses. Gzms and perf could not be detected in granulocytes previously activated either in vitro and/or in vivo, by L monocytogenes or by
pathogen-derived material, such as LPS, Lip-OspA, or the yeast
product, zymosan. This indicates that mouse granulocytes do not use the
known NK/CTL-associated cytotoxic effector molecules, gzmA, gzmB,
and perf, as defense against pathogens and tumors. Our finding that
fractions of CD4⫹ and CD8⫹ T cells, but not highly purified PMNs from
4 patients and 3 healthy individuals, expressed gzmB is in support of
recent work from 2 independent groups,26,27 but contrasts with other
related studies.18,19 Thus, it remains to be verified whether the discrepant
results obtained using human PMNs are merely due to technical
differences and whether mouse and/or human PMNs or fractions thereof
are able to produce gzms and/or perf, but only under stringent conditions
unlike the expression in NK/CTLs.
Acknowledgments
We thank Jero Calafat from The Netherlands Cancer Institute,
Division of Cell Biology, Amsterdam, for performing the IEM
experiments and for her critical comments, and Verena Weber,
Claudia Luckner, Christiane Brenner, and Aynur Ekiciler for expert
technical assistance.
References
1. Kagi D, Ledermann B, Burki K, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:
31-37.
2. Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin
and Fas lytic pathways. Nature. 1994;370:650-652.
3. Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require
granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target
cells. Cell. 1994;76:977-987.
4. Simon MM, Hausmann M, Tran T, et al. In vitro–
and ex vivo–derived cytolytic leukocytes from
granzyme A x B double knockout mice are defective in granule-mediated apoptosis but not lysis of
target cells. J Exp Med. 1997;186:1781-1786.
5. Balkow S, Kersten A, Tran TT, et al. Concerted
action of the FasL/Fas and perforin/granzyme A
and B pathways is mandatory for the development of early viral hepatitis but not for recovery
from viral infection. J Virol. 2001;75:8781-8791.
ase 1 (TSP-1) reveals two types of L3T4⫹ T lymphocytes. Eur J Immunol. 1988;18:773-781.
14. Zheng LM, Ojcius DM, Liu CC, et al. Immunogold
labeling of perforin and serine esterases in granulated metrial gland cells. FASEB J. 1991;5:79-85.
15. Garcia-Sanz JA, MacDonald HR, Jenne DE,
Tschopp J, Nabholz M. Cell specificity of granzyme gene expression. J Immunol. 1990;145:
3111-3118.
16. Sayers TJ, Brooks AD, Ward JM, et al. The restricted expression of granzyme M in human lymphocytes. J Immunol. 2001;166:765-771.
17. Sedelies KA, Sayers TJ, Edwards KM, et al. Discordant regulation of granzyme H and granzyme
B expression in human lymphocytes. J Biol
Chem. 2004;279:26581-26587.
18. Wagner C, Iking-Konert C, Denefleh B, Stegmaier
S, Hug F, Hansch GM. Granzyme B and perforin:
constitutive expression in human polymorphonuclear neutrophils. Blood. 2004;103:1099-1104.
6. Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323-370.
19. Hochegger K, Eller P, Rosenkranz AR. Granzyme
A: an additional weapon of human polymorphonuclear neutrophils (PMNs) in innate immunity?
[letter]. Blood. 2004;103:1176.
7. Trapani JA, Sutton VR. Granzyme B: pro-apoptotic,
antiviral and antitumor functions. Curr Opin Immunol.
2003;15:533-543.
20. Grossman WJ, Revell PA, Lu ZH, Johnson H, Bredemeyer AJ, Ley TJ. The orphan granzymes of humans
and mice. Curr Opin Immunol. 2003;15:544-552.
8. Pardo J, Bosque A, Brehm R, et al. Apoptotic
pathways are selectively activated by granzyme A
and/or granzyme B in CTL-mediated target cell
lysis. J Cell Biol. 2004;167:457-468.
21. Catalfamo M, Henkart PA. Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin
Immunol. 2003;15:522-527.
9. Müllbacher A, Waring P, Hia RT, et al. Granzymes
are the essential downstream effector molecules
for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci U S A. 1999;
96:13950-13955.
10. Lieberman J, Fan Z. Nuclear war: the granzyme
A-bomb. Curr Opin Immunol. 2003;15:553-559.
11. Fruth U, Sinigaglia F, Schlesier M, Kilgus J,
Kramer MD, Simon MM. A novel serine proteinase (HuTSP) isolated from a cloned human CD8⫹
cytolytic T cell line is expressed and secreted by
activated CD4⫹ and CD8⫹ lymphocytes. Eur
J Immunol. 1987;17:1625-1633.
12. Simon HG, Fruth U, Eckerskorn C, et al. Induction of T cell serine proteinase 1 (TSP-1)–specific
mRNA in mouse T lymphocytes. Eur J Immunol.
1988;18:855-861.
13. Fruth U, Nerz G, Prester M, Simon HG, Kramer
MD, Simon MM. Determination of frequency of T
cells expressing the T cell-specific serine protein-
22. Kagan BL, Ganz T, Lehrer RI. Defensins: a family
of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:131-149.
23. Levy O. Antibiotic proteins of polymorphonuclear
leukocytes. Eur J Haematol. 1996;56:263-277.
24. van Kessel KP, Verhoef J. A view to a kill: cytotoxic mechanisms of human polymorphonuclear
leukocytes compared with monocytes and natural
killer cells. Pathobiology. 1990;58:249-264.
25. van Egmond M, van Spriel AB, Vermeulen H,
Huls G, van Garderen E, van de Winkel JG. Enhancement of polymorphonuclear cell-mediated
tumor cell killing on simultaneous engagement of
fcgammaRI (CD64) and fcalphaRI (CD89). Cancer Res. 2001;61:4055-4060.
26. Metkar SS, Froelich CJ. Human neutrophils lack
granzyme A, granzyme B, and perforin [letter].
Blood. 2004;104:905-906; author reply 907-908.
27. Grossman WJ, Ley TJ. Granzymes A and B are
not expressed in human neutrophils [letter].
Blood. 2004;104:906-907; author reply 907-908.
28. Ebnet K, Chluba-de Tapia J, Hurtenbach U,
Kramer MD, Simon MM. In vivo primed mouse T
cells selectively express T cell-specific serine proteinase-1 and the proteinase-like molecules granzyme B and C. Int Immunol. 1991;3:9-19.
29. Pardo J, Balkow S, Anel A, Simon MM. The differential contribution of granzyme A and granzyme B
in cytotoxic T lymphocyte-mediated apoptosis is
determined by the quality of target cells. Eur J Immunol. 2002;32:1980-1985.
30. Ebnet K, Hausmann M, Lehmann-Grube F, et
al. Granzyme A-deficient mice retain potent cellmediated cytotoxicity. EMBO J. 1995;14:4230-4239.
31. Rode M, Balkow S, Sobek V, et al. Perforin and
Fas act together in the induction of apoptosis,
and both are critical in the clearance of lymphocytic choriomeningitis virus infection. J Virol.
2004;78:12395-12405.
32. Calafat J, Janssen H, Stahle-Backdahl M, Zuurbier AE, Knol EF, Egesten A. Human monocytes
and neutrophils store transforming growth factor-␣ in a subpopulation of cytoplasmic granules.
Blood. 1997;90:1255-1266.
33. Liou W, Geuze HJ, Slot JW. Improving structural
integrity of cryosections for immunogold labeling.
Histochem Cell Biol. 1996;106:41-58.
34. Slot JW, Geuze HJ, Gigengack S, Lienhard GE,
James DE. Immuno-localization of the insulin
regulatable glucose transporter in brown adipose
tissue of the rat. J Cell Biol. 1991;113:123-135.
35. Simon MM, Hoschutzky H, Fruth U, Simon HG,
Kramer MD. Purification and characterization of a T
cell specific serine proteinase (TSP-1) from cloned
cytolytic T lymphocytes. EMBO J. 1986;5:3267-3274.
36. Hestdal K, Ruscetti FW, Ihle JN, et al. Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22-28.
37. Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes.
J Immunol Methods. 1996;197:139-150.
38. Kaech SM, Tan JT, Wherry EJ, Konieczny BT,
Surh CD, Ahmed R. Selective expression of the
interleukin 7 receptor identifies effector CD8 T
cells that give rise to long-lived memory cells.
Nat Immunol. 2003;4:1191-1198.
39. Baggiolini M, Schnyder J, Bretz U, Dewald B, Ruch
W. Cellular mechanisms of proteinase release from
inflammatory cells and the degradation of extracellular proteins. Ciba Found Symp. 1979:105-121.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2005 106: 2871-2878
doi:10.1182/blood-2005-04-1522 originally published online
July 5, 2005
Quiescent and activated mouse granulocytes do not express granzyme
A and B or perforin: similarities or differences with human
polymorphonuclear leukocytes?
Praxedis Martin, Reinhard Wallich, Julian Pardo, Arno Müllbacher, Markus Munder, Manuel Modolell
and Markus M. Simon
Updated information and services can be found at:
http://www.bloodjournal.org/content/106/8/2871.full.html
Articles on similar topics can be found in the following Blood collections
Phagocytes (974 articles)
Information about reproducing this article in parts or in its entirety may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests
Information about ordering reprints may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#reprints
Information about subscriptions and ASH membership may be found online at:
http://www.bloodjournal.org/site/subscriptions/index.xhtml
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society
of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Copyright 2011 by The American Society of Hematology; all rights reserved.