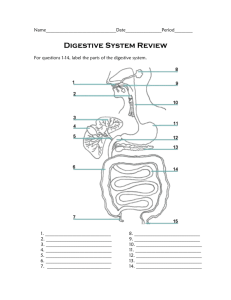
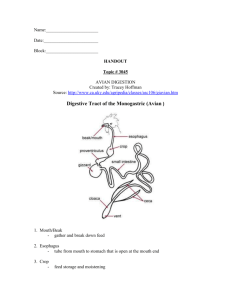
Laboratory 11 Digestive System
advertisement

Lab 11 Digestive System Laboratory 11 Digestive System Background The digestive system is a portal for nutrients from the environment to gain access to the circulatory system. Before that transfer can occur, food first has to be reduced to very simple molecules by a combination of mechanical and enzymatic degradation. The resulting sugar, amino acids, fatty acids, and other nutrient molecules can be transported across the epithelium lining the intestine into the blood. Objective: To examine and understand the big picture of digestive physiology Review of Food Chemistry The diet of any animal contains hundreds, if not thousands, of different molecules. However, the bulk of the ingested nutrients are in the form of huge macromolecules that cannot be absorbed into blood (remember diffusion and osmosis) without first being reduced to simpler and smaller forms. Even table sugar (sucrose) cannot be absorbed without first being enzymatically broken down into glucose and fructose. The most important enzymatic reaction in digestion of foodstuffs is hydrolysis - the breaking of a chemical bond by the addition of a water molecule. Proteins Proteins are polymers of amino acids linked together by peptide bonds. Chain length varies tremendously and many dietary proteins have been modified after translation by addition of carbohydrate (glycoproteins) or lipid (lipoprotein) moieties. Very short proteins, typically 3 to 10 amino acids in length, are called peptides. Although very small peptides can be absorbed to a limited degree, for all intents and purposes, proteins must be reduced to single amino acids before they can be absorbed. Enzymes that hydrolyze peptide bonds and reduce proteins or peptides to amino acids are called proteases or peptidases. Lipids Fatty acids are present in only small amounts in animal and plant tissues, but are the building blocks of many important complex lipids. True fatty acids possess a long hydrocarbon chain terminating in a carboxyl group. Nearly all fatty acids have an even number of carbons and have chains between 14 and 22 carbons in length. The principle differences among the many fatty acids are the length of the chain (usually 16 or 18 carbons) and the positions of unsaturated or double bonds. For example, stearic acid has 18 carbons and is saturated. The so-called "short-chain" or volatile fatty acids are 2 to 4-carbon molecules of great importance in intermediary metabolism and as the mainstay of ruminant nutrition. They are represented by acetic, butyric and proprionic acids. The most abundant storage form of fat in animals and plants, and hence the most important dietary lipid, is neutral fat or triglyceride. A molecule of triglyceride is composed of a molecule of glycerol in which each of the three carbons is linked through an ester bond to a fatty acid. Triglycerides cannot be efficiently absorbed, and are enzymatically digested by pancreatic lipase into a 2-monoglyceride and two free fatty acids, all of which can be absorbed. Other lipases hydrolyze a triglyceride into glycerol and three fatty acids. 1 Lab 11 Digestive System Carbohydrates The diversity of dietary carbohydrates necessitates discussion of several classes of these molecules, ranging from simple sugars to huge, branched polymers. Monosaccharides or simple sugars are either hexoses (6-carbon) like glucose, galactose and fructose, or pentoses (5-carbon) like ribose. These are the breakdown products of more complex carbohydrates and can be efficiently absorbed across the wall of the digestive tube and transported into blood. Disaccharides are simply two monosaccharides linked together by a glycosidic bond. The disaccharides most important in nutrition and digestion are: • lactose or "milk sugar": glucose + galactose • sucrose or "table sugar": glucose + fructose • maltose: glucose + glucose Oligosaccharides, which include disaccharides, are relatively short chains of monosaccharides that are typically intermediates in the breakdown of polysaccharides to monosaccharides. Polysaccharides are the most abundant dietary carbohydrate for all except very young animals. You should be familiar with three important polysaccharides, each of which is a large polymer of glucose: • Starch is a major plant storage form of glucose. It occurs in two forms: 1) alpha-amylose, in which the glucoses are linked together in straight chains, and 2) amylopectin, in which the glucose chains are highly branched. Except for the branch points of amylopectin, the glucose monomers in starch are linked via alpha(1-4) glycosidic bonds, which, in the digestive tract of mammals, are hydrolyzed by amylases. • Cellulose is the other major plant carbohydrate. It is the major constituent of plant cell walls, and more than half of the organic carbon on earth is found in cellulose. Cellulose is composed of unbranched, linear chains of D-glucose molecules, linked to one another by beta(1-4) glycosidic bonds, which no vertebrate has the capacity to enzymatically digest. Herbivores subsist largely on cellulose, not because they can digest it themselves, but because their digestive tracts teem with microbes that produce cellulases that hydrolyze cellulose. • Glycogen is the third large polymer of glucose and is the major animal storage carbohydrate. Like starch, the glucose molecules in glycogen are linked together by alpha(1-4) glycosidic bonds. Proceed to setup the following experiments. After the experiments are setup, work on physioEX exercise 8: Chemical and Physical Processes of Digestion (pp. 103-113). Experiment 1: Gastric Digestion Background Foodstuffs entering the stomach have been, to at least some extent, crushed and reduced in size by mastication, and partially digested by enzymes in saliva. The stomach provides four basic functions that assist in the early stages of digestion and prepare the ingesta for further processing in the small intestine: 1 It serves as a short-term storage reservoir, allowing a rather large meal to be consumed quickly and dealt with over an extended period of time. 2 It is in the stomach that substantial enzymatic digestion is initiated, particularly of proteins. 2 Lab 11 Digestive System 3 Vigorous contractions of gastric smooth muscle mix and grind foodstuffs with gastric secretions, resulting in liquefaction of food, a prerequisite for delivery of the ingesta to the small intestine. 4 As food is liquefied in the stomach, it is slowly released into the small intestine for further processing. During gastric digestion, glands in the stomach wall secrete gastric juice, which mixes with food particles. Gastric juice is a mixture of hydrochloric acid and the enzyme, pepsin. At body temperature (37ºC) and in the presence of hydrochloric acid, pepsin reduces proteins into amino acids. The food particles are converted to a semi-liquid state in preparation for intestinal digestion, where enzyme break-down the particles even more. Safety Note: Use caution when handling hydrochloric acid and artificial gastric juice. Biuret reagent can stain skin and clothes. Procedure: 1. Obtain three test tubes. Number them 1, 2, and 3. 2. Place one scoop of powdered albumin in each test tube. 3. Using graduated cups: a. Add 5 mL of pepsin solution to Test Tube 1. b. Add 5 mL of hydrochloric acid to Test Tube 2. c. Add 5 mL of artificial gastric juice (containing pepsin and hydrochloric acid) to Test Tube 3. 4. Place the test tubes in a warm water bath (37-40ºC) for 45 minutes. 5. Remove the test tubes from the water bath. Add six drops of Biuret reagent to each test tube. Shake each test tube to mix the contents. Record the colors below. For the best view, look downward through the top of each test tube. Table 1. Gastric digestion: color of solutions after adding Biuret reagent Test Tube No. Substrate Enzyme Other Color 1 2 3 Biuret reagent reacts with the products of gastric protein digestion (amino acids) to give a pinkviolet color. a. What is the role of hydrochloric acid? b. In which test tube(s) did protein digestion occur? 3 Lab 11 Digestive System c. Why is the water bath temperature set to 37-40ºC? d. How does pH affect the efficiency of pepsin? The small intestine is the portal for absorption of virtually all nutrients into blood. Accomplishing this transport entails breaking down large supramolecular aggregates into small molecules that can be transported across the epithelium. By the time ingesta reaches the small intestine, foodstuffs have been mechanically broken down and reduced to a liquid by mastication and grinding in the stomach. Once within the small intestine, these macromolecular aggregates are exposed to pancreatic enzymes and bile, which enables digestion to molecules capable or almost capable of being absorbed. The final stages of digestion occur on the surface of the small intestinal epithelium. The net effect of passage through the small intestine is absorption of most of the water and electrolytes (sodium, chloride, potassium) and essentially all dietary organic molecules (including glucose, amino acids and fatty acids). Through these activities, the small intestine not only provides nutrients to the body, but plays a critical role in water and acid-base balance. Dietary Polysaccharides: Structure and Digestion Polysaccharides, particularly of plant origin, are prominent components in the diets are herbivores and omnivores. This complex set of molecules has been categorized in several ways, depending on whether the focus is chemistry or nutrition. From the standpoint of digestive physiology and nutrition, perhaps the most relevant classification is based on whether or not animals synthesize enzymes that allow the polysaccharide in question to be digested into absorbable monosaccharides. In this view, we have starch, which can be digested by vertebrate enzymes, versus fiber, which cannot. Starch: Amylose and Amylopectin Starch is the principle carbohydrate found in plant seeds and tubers; important sources of starch include maize (corn), potato and rice. Starch exists in the form of granules, each of which consists of several million amylopectin molecules together with an even larger number of amylose molecules. Since amylopectin is a much larger molecule than amylose, the mass of amylopectin is typically 4 to 5 times that of amylose in starch. Amylose consists of linear, helical chains of roughly 500 to 20,000 alpha-D-glucose monomers linked together through alpha (1-4) glycosidic bonds. Amylopectin molecules are huge, branched polymers of glucose, each containing between one and two million residues. In contrast to amylose, amylopectin is branched. It contains numerous amylose-like chains of up to 30 glucose residues linked through alpha (1-4) bonds, connected to one another through alpha (1-6) branch points. Starch is digested to glucose in two basic steps: First amylose and amylopectin are hydrolyzed into small fragments through the action of alphaamylase, secreted by salivary glands in some species, and from the pancreas in all. Amylase cleaves only internal alpha (1-4) glycosidic bonds, thereby reducing starch to three different oligosaccharides: maltose (disaccharide), maltotriose (trisaccharide), and a group of alpha-limit dextrins, which contain branch points from amylopectin. 4 Lab 11 Digestive System Second, maltose, maltotriose and limit dextrins are hydrolyzed on the lumenal surface of the small intestine by a brush border enzyme complex called sucrase-isomaltase (also often referred to as maltase). This step ultimately yields glucose monomers that are then transported into the small intestinal enterocyte by co-transport with sodium ions. Dietary Fiber: Cellulose and Hemicellulose Several definitions have been proposed for "fiber". An early definition, still quite appropriate, basically states that fiber is the portion of food derived from plant cell walls that is poorly digested by mammals. To put it another way, mammals often consume fiber, but do not themselves secrete the enzymes necessary to digest it into a form that can be absorbed. Another common definition for fiber is the non-starch polysaccharide component of foodstuffs. The chief components of dietary fiber are cellulose and hemicellulose, both of plant origin. Pectin and pectic acid are other plant polysaccharides often present in diets. Cellulose is a linear polymer of between 1000 and 10,000 beta-D-glucose molecules in which adjacent glucose molecules are joined covalently through beta (1-4) glycosidic bonds. The beta (1-4) bonds cause the polymer to assume a non-helical, straight structure, which is different from the helical structure imposed on starch molecules by the alpha (1-4) bonding. The non-helical structure of cellulose also promotes hydrogen bonding between cellulose molecules. Cellulose polymers associate with one another through a huge number of hydrogen bonds to form microfibers. Microfibers interact to form cellulose fibers. A typical fiber contains roughly 500,000 cellulose molecules. The high tensile strength of cellulose fibers reflects the massive number of hydrogen bonds involved in its structure. Hemicellulose is a heteropolymer composed of a variety of sugars, including xylose, arabinose and mannose in a branched structure. In contrast to the highly ordered structure of cellulose, hemicellulose assumes an amorphose structure and becomes highly hydrated to form a gel. No vertebrate cell has been identified that produces an enzyme that hydrolyzes celluloses or hemicelluloses. Certainly, amylase will not cleave these two polysaccharides. Dietary fiber therefore is indigestible and passes through the small intestine essentially unchanged. Within the large intestine, fiber is digested by enzymes - cellulases and hemicellulases - of microbial origin, a process referred to as fermentation. Fermentation does not yield monosaccharides that can be absorbed. Rather, its chief products are short chain volatile fatty acids, which are readily absorbed and utilized for energy and lipid synthesis. Thus, if the fermentation vat is of sufficient size (i.e. herbivores), dietary fiber can be a major source of energy. Experiment 2: Intestinal Digestion. Digestion of Starch Background The semi-liquid produced during gastric digestion passes into the small intestine, stimulating the production of alkaline intestinal fluids. When the semi-liquid becomes alkaline, gastric digestion ceases and intestinal digestion begins. Pancreatic juice is secreted into the duodenum. This juice contains the enzyme amylase, which breaks starch into glucose, which can be absorbed into the body through the lining of the small intestine. Safety Note: Use caution when handling iodine-potassium iodide solution. It can stain skin and clothes. 5 Lab 11 Digestive System Procedure: 1. 2. 3. 4. 5. Obtain three test tubes. Number them 1, 2, and 3. Shake the starch solution well. Add 5 mL. of starch solution to each test tube. Add 5 mL of pancreatic juice to each test tube. Gently shake each test tube to mix the contents. For 30 minutes, maintain the test tubes separately under the following conditions: Test Tube 1: room temperature Test Tube 2: in a 40ºC water bath Test Tube 3: in a 70ºC water bath 6. Remove Test Tube 2 and Test Tube 3 from the water baths. Add three drops of iodinepotassium iodide solution to all three test tubes. Gently shake each test tube to mix the contents. Record the colors below. For the best view, look downward through the top of each test tube. Table 2. Intestinal Digestion of Starch: color of solutions after adding iodine-potassium iodide solution Test Tube No. Substrate Enzyme Temperature Color 1 2 3 In the presence of starches, iodine-potassium iodide changes from bright yellow to blue-black. In the test tubes, the presence of starch is indicated by a purple-black color. The absence of starch is indicated by a light brown color. a. What happened to the starch in Test Tube 1? Has digestion occurred? b. What happened to the starch in Test Tube 2? Has digestion occurred? c. What happened to the starch in Test Tube 3? Has digestion occurred? d. What is the product of starch digestion? e. Which temperature was best for starch digestion? Why do you think is so? 6 Lab 11 Digestive System Experiment 3: Intestinal Digestion: Digestion of Proteins and Lipids. Background The semi-liquid produced during gastric digestion passes into the small intestine, stimulating the production of alkaline intestinal fluids. When the semi-liquid becomes alkaline, gastric digestion ceases and intestinal digestion begins. Juice is secreted by the pancreas into the duodenum. This pancreatic juice contains the enzymes trypsin and lipase. Proteins are broken down into amino acids by trypsin. Fats are broken down into glycerol and fatty acids by lipase. Amino acids and fatty acids can be absorbed into the body through the lining of the small intestine. Procedure: 1. Obtain two test tubes. Number them 1, and 2. 2. Add 10 mL of litmus milk solution to each test tube. 3. To Test Tube 1, add one scoop of pancreatin powder. Shake the test tube gently to mix the contents. 4. To Test Tube 2, add a few drops of distilled water. Shake the test tube gently to mix the contents. 5. Place both of the test tubes in a warm water bath (37-40ºC) for 45 minutes. 6. Remove the test tubes and observe the colors. Record the colors below. For the best view, look downward through the top of each test tube. Table 3. Intestinal Digestion of Proteins and Lipids: color of solutions after incubation Test Tube No. Substrates Enzymes Color 1 2 The milk turns blue when basic and red when acidic. The litmus milk indicates the presence of acid by a color change from light gray to dark pink-purple. a. What happened to the proteins and lipids (fats and oils) in Test Tube 1? Has digestion occurred? b. What happened to the proteins and lipids (fats and oils) in Test Tube 2? Has digestion occurred? c. Milk contains proteins and lipids (fats and oils). Why would the digestion of this nutrients cause an increase in acidity? d. Why is the bath water temperature set to 37-40ºC? 7 Lab 11 Digestive System Experiment 4: Absorption in the Small Intestine. Background To provide nourishment useful to the body cells, absorption must follow gastric and intestinal digestion. Gastric digestion reduces food particles to a semi-liquid state. Intestinal digestion breaks down starches into glucose, and proteins and fats into amino acids and fatty acids. Absorption is the passage of digestion byproducts through the intestinal mucosa into the blood or lymph. If molecules are not broken down adequately, they are not absorbed through the lining of the small intestine. Procedure: 1. Soak and eight-inch piece of dialysis tubing in distilled water for two minutes. Roll the dialysis tubing between your thumb and index finger to open it. 2. Close one end of the dialysis tubing with a clamp to create a bag. 3. Half-fill the dialysis tubing with an equal mixture of starch solution and glucose solution. Close the other of the tubing with a clamp, making sure there are no bubbles in the bag. 4. Rinse the outside of the tubing with tap water. Carefully wipe dry the dialysis tubing to remove traces of nutrients. 5. Place the tubing in a 250-mL beaker containing 50 mL of distilled water. Wait 30 minutes. During this time, make sure that the dialysis tubing remains completely submerged in the distilled water. 6. Obtain two test tubes. Number them 1, and 2. 7. Remove the dialysis tubing from the beaker. Using a graduated cup, transfer 5 mL of the water in the beaker into each test tube. 8. Add 20 drops of Benedict’s solution to Test Tube 1. Place the test tube in a boiling water bath for five minutes. After five minutes observe the color. When heated, Benedict’s solution reacts with glucose to produce a red-orange precipitate. a. From your observation of the test tube, did glucose diffuse into the water in the beaker? How do you know? b. Do you think glucose can be absorbed by the small intestine? Why or why not? 9. Add three drops of iodine-potassium iodide to Test Tube 2. Observe the color. The presence of starch is indicated by a blue-black color. a. From your observation of the test tube, did starch diffuse into the water in the beaker? b. Do you think starch can be absorbed by the small intestine? Why or why not? 8